Visão geral
A timosina beta 4 (Tβ4) é uma proteína de pequena molécula composta por múltiplos resíduos de aminoácidos e está amplamente distribuída em vários tecidos e células do corpo humano.[1][2] Como uma das principais moléculas reguladoras da actina no corpo humano, tem múltiplas funções biológicas e desempenha um papel crucial na regeneração dos tecidos, remodelação, cicatrização de feridas, manutenção do equilíbrio da actina, desenvolvimento de tumores e metástases, apoptose celular, inflamação, angiogénese, desenvolvimento do folículo piloso e outros processos fisiológicos e patológicos.
Funções biológicas e mecanismos de ação
A Tβ4 é uma das principais moléculas reguladoras da actina no corpo humano e tem múltiplas funções biológicas, desempenhando um papel significativo na regeneração dos tecidos, na remodelação, na cicatrização de feridas, na manutenção do equilíbrio da actina, no desenvolvimento de tumores e metástases, na apoptose celular, na inflamação, na angiogénese, no desenvolvimento do folículo piloso e noutros processos fisiológicos e patológicos.
Fonte: Tandfonline
- Fator regulador da actina: A actina é responsável por cerca de 10% da proteína total em células não musculares e é um componente essencial necessário para a estrutura celular, movimento celular e cicatrização de feridas. A presença de Tβ4 nas células é suficiente para sequestrar todos os monómeros de actina e participar na regulação da polimerização e despolimerização da actina. O Tβ4 pode ligar-se aos monómeros de actina numa proporção de 1:1, impedindo a formação de polímeros de F-actina. A ligação de Tβ4 à actina é acompanhada pela dissociação da água ligada. O terminal C de Tβ4 liga-se ao His-40 da actina, provocando uma alteração conformacional nos monómeros de actina. A molécula de Tβ4 contém um domínio de ligação à actina (LKKTET), que é o principal local de contacto eletrostático. O seu fragmento N-terminal pode inibir a polimerização da actina por impedimento estérico. Quando actua isoladamente, a Tβ4 pode inibir a polimerização da actina e a troca de nucleótidos na actina. Isto contrasta com o papel de outra proteína de ligação à actina, a Profilin, que promove a troca de ADP e ATP, acelerando a montagem da actina. [1][2]A Tβ4 e a Profilina podem regular sinergicamente a montagem da actina. A Tβ4 pode regular a conversão entre a G-actina e a F-actina. Foi relatado que a concentração de Tβ4 atinge 300 pmol em células sanguíneas altamente móveis, enquanto a concentração necessária para que Tβ4 se ligue à G-actina é inferior a 20 pmol. Com um aumento da concentração, o Tβ4 reduz a capacidade de despolimerização da F-actina. Esta pode ser a razão pela qual o Tβ4 regula a função do sistema de microfilamentos celulares.
Promoção da migração das células endoteliais e da angiogénese: Estudos realizados com o modelo de angiogénese da membrana corioalantóica de galinha (CAM) demonstraram que, quando as células endoteliais se diferenciam em estruturas tubulares, o teor de ARNm de Tβ4 aumenta cinco vezes e a transfecção com Tβ4 acelera a formação de estruturas semelhantes a tubos em clones de células endoteliais. Além disso, a investigação descobriu que o Tβ4 pode interagir com a fibrina e o colagénio mediados pela transglutaminase (Fator XIIIa) e desempenhar um papel importante no processo de coagulação do sangue. Além disso, numa experiência de lesão cutânea de espessura total em ratos diabéticos envelhecidos, o Tβ4 pode acelerar a cicatrização de feridas cutâneas de grande área e queimaduras profundas, promover a reparação da pele e da córnea e demonstrar a capacidade de acelerar a cicatrização de feridas.
Inibição da apoptose celular: A Tβ4 tem um efeito protetor significativo nas células epiteliais da córnea danificadas por substâncias corrosivas como o cloreto de benzalcónio ou o etanol. A regulação positiva da expressão do gene Tβ4 é benéfica para aumentar a resistência das células à hipoxia.[2][3]
Regulação negativa das principais moléculas inflamatórias: A cicatrização de feridas é um processo biológico complexo que pode ser dividido em três fases distintas: inflamação, proliferação celular e remodelação de tecidos, com a expressão genética de proteínas relevantes a ser regulada positivamente e depois desregulada em cada fase. No caso das feridas crónicas, este processo regulador pode ser perturbado por factores como a idade, doenças subjacentes e fármacos imunossupressores, levando à produção excessiva de moléculas inflamatórias, inflamação excessiva e impedindo a proliferação celular e a remodelação dos tecidos. A investigação descobriu que a Tβ4 pode reduzir os níveis de radicais livres, abrandar a peroxidação lipídica, inibir a produção de citocinas inflamatórias como a IL-1, a proteína inflamatória de macrófagos 1α (MIP1α), a MIP1β, a proteína quimiotáctica de monócitos-1 (MCP-1), diminuir os níveis de tromboxano e prostaglandina 2α, aliviando assim a inflamação. Por conseguinte, pode ser utilizado para o tratamento de doenças inflamatórias, como a enterite segmentar e a atrofia muscular.
Estimulação da diferenciação de células estaminais epicárdicas adultas: O Tβ4 desempenha um papel crucial em vários aspectos do desenvolvimento dos vasos coronários e pode estimular significativamente o crescimento de enxertos epicárdicos de ratinhos adultos quiescentes, restaurando a multipotência dos fibroblastos, das células musculares lisas e das células endoteliais e induzindo a sua diferenciação.[1][2] O bloqueio do gene Tβ4 no coração conduz a uma diminuição significativa dos níveis do produto de clivagem angiogénica do Tβ4 (AcSDKP). Embora a injeção de AcSDKP não possa restaurar o coração, pode aumentar significativamente a diferenciação de células progenitoras epicárdicas de ratinhos adultos em células endoteliais. Isto sugere que a Tβ4 e a AcSDKP são potentes estimuladores da neovascularização coronária e que as células epicárdicas de ratinho adulto induzidas pela Tβ4 podem servir como fonte de regeneração vascular, levando a uma regeneração sustentada da vasculatura comprometida a um nível baixo após lesão cardíaca.
Estimulação do desenvolvimento do folículo piloso: O Tβ4 promove a migração, a diferenciação e a reconstrução da matriz extracelular das células estaminais do folículo piloso, regulando assim o crescimento do cabelo. Estudos realizados em ratos e ratinhos revelaram que, durante o ciclo de crescimento do pelo, um subconjunto específico de queratinócitos do folículo piloso com origem na região do bojo co-expressa fortemente a Tβ4, enquanto as células estaminais da pele também estão presentes no bojo do folículo piloso. Quando a concentração de Tβ4 se situa ao nível nanomolar, a migração e a diferenciação das células estaminais são reforçadas, e a presença de Tβ4 também aumenta a expressão e a secreção da metaloproteinase-2 da matriz (MMP-2), uma enzima de degradação da matriz extracelular.
Relação com a formação de tumores: Foi observada a expressão do gene Tβ4 em várias células tumorais, incluindo o carcinoma medular da tiroide, o cancro colorrectal, o melanoma altamente maligno, o cancro da mama e o carcinoma oral de células escamosas. O Tβ4 pode induzir eficazmente a expressão do fator de crescimento endotelial vascular, promovendo a angiogénese e activando as propriedades migratórias das células, conduzindo à malignidade do tumor. A regulação positiva da expressão de Tβ4 resulta na regulação negativa da expressão de E-caderina, enfraquecendo a adesão celular, e no aumento da expressão de metaloproteinases da matriz, proporcionando vantagens de crescimento e caraterísticas invasivas às células cancerígenas, contribuindo assim para a malignidade. O Tβ4 possui capacidades anti-apoptóticas, e o aumento da expressão de Tβ4 pode reduzir a extensão da apoptose celular, possivelmente devido ao facto de o Tβ4 inibir a libertação de citocromo c e perturbar o início do processo de apoptose, que é outra caraterística importante dos tumores malignos.
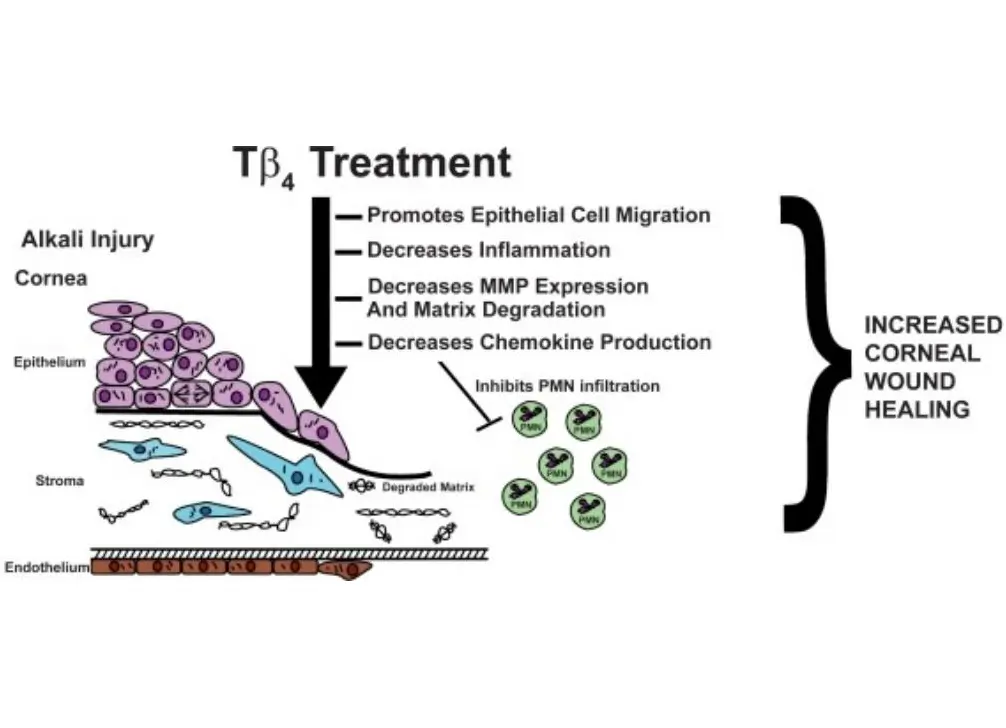
Promoção da reparação da córnea: A timosina β4 demonstrou a sua capacidade de promover a cicatrização de feridas em vários modelos de lesões da córnea e de regular a produção de determinadas citocinas-chave. A investigação descobriu que a timosina β4 pode reduzir a expressão das metaloproteinases da matriz, promover a remodelação da matriz extracelular e ativar as citocinas. Especificamente, inibe a atividade das metaloproteinases da matriz, contrariamente à conclusão retirada dos modelos de lesões cutâneas tratados com timosina β4. [1][2][3]Isto sugere que as vias reguladoras de enzimas específicas no processo de reparação de tecidos podem ser reguladas para cima ou para baixo.
Método de preparação
Atualmente, existem duas fontes principais de timosina beta 4 (Tβ4) utilizadas em contextos clínicos e de investigação: a extração do timo bovino e a síntese química. A extração de Tβ4 do timo bovino não só é dispendiosa como também é limitada pela disponibilidade de material e pelas técnicas de extração, o que resulta numa baixa pureza e num baixo teor. A presença de impurezas também traz muitos problemas, especialmente considerando o número crescente de vírus no gado, o que complica o processo de extração. Com o desenvolvimento da engenharia genética, a utilização de métodos de engenharia genética para produzir Tβ4 tornar-se-á uma nova direção. No nosso laboratório, planeamos realizar a expressão em tandem de Tβ4 sintetizado artificialmente em plantas para obter plantas transgénicas com expressão eficiente de Tβ4. Em comparação com o Tβ4 extraído naturalmente, o Tβ4 produzido com plantas transgénicas tem as vantagens de uma maior bioatividade, maior pureza e menos efeitos secundários. Além disso, representa a abordagem ideal para reduzir os custos de produção, minimizar a contaminação e permitir a produção em grande escala.[3]
Antecedentes
A timosina beta 4 é um péptido composto por 43 resíduos de aminoácidos com um ponto isoelétrico de 5,1 e é altamente conservada em mamíferos. É uma proteína citoplasmática e não uma proteína nuclear, ao contrário da timosina alfa ou de timosinas semelhantes. [2][3]A timosina beta 4 está envolvida em várias funções fisiológicas, incluindo a função imunitária, o desenvolvimento do sistema nervoso, a cicatrização de feridas e as funções da proteína actina.
Ao contrário da timosina alfa ou de timosinas semelhantes, a timosina beta 4 é uma proteína citoplasmática e não uma proteína nuclear. Contém menos aminoácidos hidrofóbicos e não contém a região estrutural Lys-Lys-Xaa-Lys. Estudos de conformação química mostraram que a timosina beta 4 existe e funciona como uma cadeia única. Tem uma região de repetição interna entre os resíduos 31-43 e 18-30, com seis aminoácidos idênticos. Embora existam duas regiões altamente helicoidais entre os resíduos 4-12 e 32-40, a timosina beta 4 não forma uma curva de prolina. Em termos de distribuição biológica e expressão, a timosina beta 4 foi inicialmente purificada a partir do timo. Por conseguinte, pensou-se inicialmente que se tratava de uma hormona tímica que actuava na fase inicial de maturação das células T. No entanto, a timosina beta 4 está amplamente presente noutros tecidos, órgãos e células, sendo os níveis mais elevados encontrados no baço, timo, pulmões e macrófagos peritoneais, seguidos do cérebro, fígado, rins, testículos e coração. Mesmo as células do sistema não reticuloendotelial, como os fibroblastos, podem sintetizar a timosina beta 4. Os timócitos têm níveis sete vezes mais elevados de ARNm da timosina beta 4 do que as células estromais tímicas, e a expressão da timosina beta 4 é observada em vários tipos de células sanguíneas. Estudos sobre o cDNA da timosina beta 4 mostraram que lhe falta um péptido de sinal.[1] Embora existam também proteínas secretadas no organismo que não possuem péptidos de sinalização, como a interleucina-1 (IL-1) e os factores de crescimento endotelial, os especialistas continuam a acreditar que é pouco provável que a timosina beta 4 seja uma proteína secretada e é mais provável que seja essencial para determinadas funções celulares básicas.
Aplicação
Investigação básica e aplicada sobre a otimização da função das células progenitoras endoteliais com timosina beta 4 (Tβ4)
A incidência e as taxas de mortalidade das doenças cardiovasculares isquémicas têm vindo a aumentar de ano para ano, constituindo uma séria ameaça para a saúde humana. A patogénese destas doenças é altamente complexa e a disfunção das células endoteliais desempenha um papel crucial no desenvolvimento das doenças cardiovasculares isquémicas. Embora as células endoteliais maduras possam reparar os danos endoteliais, a sua capacidade de regeneração é limitada. As células progenitoras endoteliais (EPC) são um tipo de célula precursora das células endoteliais vasculares e têm o potencial de se diferenciar em células endoteliais. Numerosos estudos demonstraram que as EPC desempenham vários papéis na reparação vascular e na neovascularização. No entanto, a aplicação clínica do transplante de EPCs ainda enfrenta muitos desafios. Várias doenças cardiovasculares ou factores de risco como o envelhecimento, a hipertensão, a hipercolesterolemia e a diabetes podem diminuir o número de EPCs circulantes e prejudicar a sua função, limitando grandemente a sua aplicação em doenças cardiovasculares isquémicas. Por conseguinte, melhorar a função das EPCs será uma estratégia importante para a futura terapia de transplante de EPCs.[1][2]
A timosina beta 4 (Tβ4), uma proteína de baixo peso molecular composta por 43 resíduos de aminoácidos, está envolvida na mediação de várias respostas biológicas, como a angiogénese, a cicatrização de feridas e o controlo da inflamação. A nossa investigação anterior revelou que a Tβ4 pode aumentar a proliferação e a migração de EPCs do sangue periférico humano, inibindo simultaneamente a sua apoptose e senescência. O Tβ4 aumenta significativamente a capacidade angiogénica das EPCs, mostrando uma relação dose-dependente com um efeito máximo a 1000 ng/mL (em comparação com o grupo de controlo, 33,33±1,86 vs 18,34±2,02, P<0,05). A análise por Western blot mostra que o Tβ4 promove a fosforilação da Akt Ser473 e da eNOS Ser1177, apresentando também uma relação dose-dependente. O siRNA da Akt e o siRNA da eNOS inibem significativamente os efeitos pró-angiogénicos do Tβ4 nas EPCs.
- Tratamento de feridas cutâneas
- Tratamento de lesões cardiovasculares e cerebrovasculares
- Tratamento de lesões oftálmicas
- Outras aplicações
Devido às suas funções biológicas, incluindo a promoção da migração das células endoteliais, a prevenção da apoptose celular, os efeitos anti-inflamatórios e a promoção do desenvolvimento do folículo piloso, a Tβ4 também demonstra um potencial significativo nos cuidados da pele, combatendo o envelhecimento da pele e promovendo o crescimento do cabelo, entre outros aspectos dos cuidados de saúde.

