Peptide Modifications
Peptide Pegylation
What is Peptide Pegylation?
Peptide polyethylene glycolisation is the process of covalently attaching one or more polyethylene glycol (PEG) chains to a peptide molecule. Polyethylene glycolisation increases the molecular weight of the peptide, protects the peptide from hydrolysis by protein hydrolases, and ultimately improves the pharmacokinetics of the peptide.
Peptides play a vital role in the pharmaceutical industry and drug therapy development. However, the in vivo application of peptides is limited due to rapid degradation by proteolytic enzymes, poor solubility, antigenic reactions, and glomerular filtration in the kidney. Covalent linkage of polyethylene glycol (PEG) reduces immunogenicity, improves solubility and reduces renal clearance. The addition of monodisperse PEG chains is a key factor in the therapeutic development of PEGylated peptides. These safe and effective polyethylene glycolated peptides are essential for optimal therapeutic efficacy.

Peptide Pegylation scientists
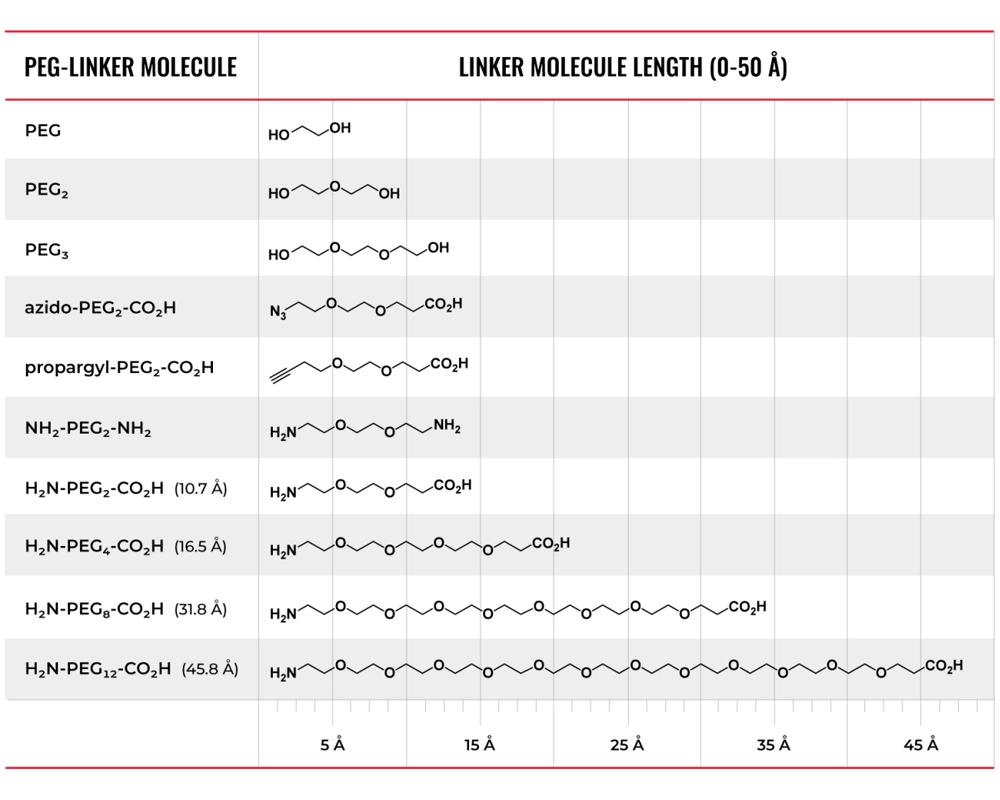
Common PEG linkers and abbreviations
·Click Chemistry, which takes place between an azide group of the PEG reagent and an alkyne group of the peptide, or vice versa.
·Suzuki-Miyaura Coupling, which takes place between the iodophenyl group of the PEG reagent and an aryl boronic acid group of peptide, or vice versa..
·Sonogashira Coupling, which takes place between an iodophenyl group of the PEG reagent and an alkyne group of the peptide, or vice versa.
|
Abbrev.
|
Full PEG Chain Name
|
|---|---|
|
PEG |
tetraethylene glycol |
|
mini-PEG2 |
8-amino-3,6-dioxaoctanoic acid |
|
mini-PEG3 |
amino-3,6,9-trioxaundecanoic acid |
|
NH2-PEG2-acid |
3-(2-(2-Aminoethoxy)ethoxy)-propanoic acid |
|
PEG750 |
Poly(ethylene glycol) methyl ether (average Mn 750) |
|
PEG1000 |
Poly(ethylene glycol) methyl ether (average Mn 1000) |
|
PEG2000 |
Poly(ethylene glycol) methyl ether (average Mn 2000) |
|
PEG5000 |
Poly(ethylene glycol) methyl ether (average Mn 5000) |
|
Ester (OTBzl) |
p-Nitroanilide |
|
KLH (-COOH of C terminal) |
tBu |
Featured Citations
Development of a Radiolabeled Irreversible Peptide Ligand for PET Imaging of Vascular Endothelial Growth Factor
Imaging agents based on peptide probes have desirable pharmacokinetic properties provided that they have high affinities for their target in vivo. An approach to improve a peptide ligand’s affinity for its target is to make this interaction covalent and irreversible. For this purpose, we evaluated a 64Cu-labeled affinity peptide tag, 64Cu-L19K-(5-fluoro-2,4-dinitrobenzene) (64Cu-L19K-FDNB), which binds covalently and irreversibly to vascular endothelial growth factor (VEGF) as a PET imaging agent. We compared the in vivo properties of 64Cu-L19K-FDNB in VEGF-expressing tumor xenografts with its noncovalent binding analogs, 64Cu-L19K-(2,4-dinitrophenyl) (64Cu-L19K-DNP) and 64Cu-L19K. Methods: The L19K peptide (GGNECDIARMWEWECFERK-CONH2) was constructed with 1,4,7-triazacyclononane-1,4,7-triacetic acid at the N terminus for radiolabeling with 64Cu with a polyethylene glycol spacer between peptide and chelate. 1,5-difluoro-2,4-dinitrobenzene was conjugated at the C-terminal lysine for cross-linking to VEGF, resulting in L19K-FDNB. 64Cu-L19K-FDNB was assayed for covalent binding to VEGF in vitro. As a control, L19K was conjugated to 1-fluoro-2,4-dinitrobenzene, resulting in L19K-DNP. PET imaging and biodistribution studies of 64Cu-L19K-FDNB, 64Cu-L19K-DNP, and the native 64Cu-L19K were compared in HCT-116 xenografts. Blocking studies of 64Cu-L19K-FDNB was performed with a coinjection of excess unlabeled L19K-FDNB. Results: In vitro binding studies confirmed the covalent and irreversible binding of 64Cu-L19K-FDNB to VEGF, whereas 64Cu-L19K-DNP and 64Cu-L19K did not bind covalently. PET imaging showed higher tumor uptake with 64Cu-L19K-FDNB than with 64Cu-L19K-DNP and 64Cu-L19K, with mean standardized uptake values of 0.62 ± 0.05, 0.18 ± 0.06, and 0.34 ± 0.14, respectively, at 24 h after injection (P < 0.05), and 0.53 ± 0.05, 0.32 ± 0.14, and 0.30 ± 0.09, respectively, at 48 h after injection (P < 0.05). Blocking studies with 64Cu-L19K-FDNB in the presence of excess unlabeled peptide showed a 53% reduction in tumor uptake at 48 h after injection. Conclusion: In this proof-of-concept study, the use of a covalent binding peptide ligand against VEGF improves tracer accumulation at the tumor site in vivo, compared with its noncovalent binding peptide analogs. This technique is a promising tool to enhance the potency of peptide probes as imaging agents.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.
