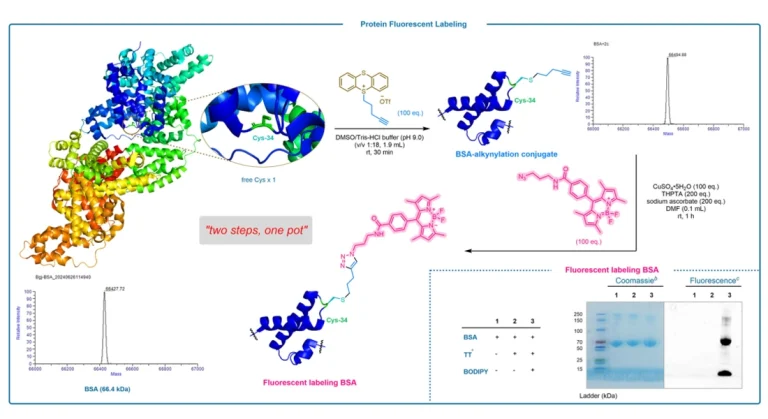
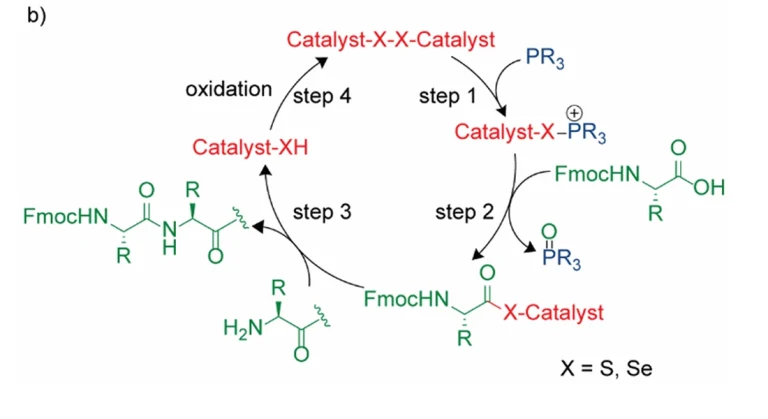
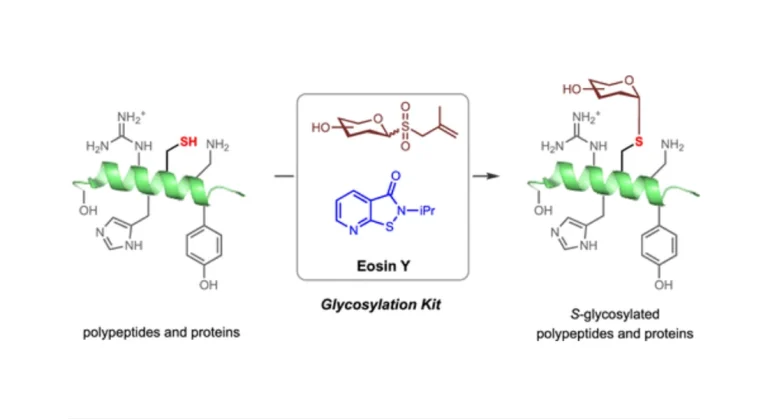
Peptides are important participants in life activities, and their specific modification and labeling are essential for studying their structure and function. Over the past decade, methods have been developed to label glycine on α-carbon. However, these methods require pre-functionalization of glycine, which is not possible for rapid recognition of natural amino acids.In this study, we report the dual activation of the α-methylene of glycine, which distinguishes this glycine from 19 other amino acids.
Technical Advantages
Specific selectivity, utilizing the unique chemical properties of N-terminal glycine to realize its specific reaction with modifying reagents and avoid the interference of other amino acid residues.
- Universal: Applicable to a wide range of N-terminal glycine-containing peptides, as well as different modification groups and fluorescent dyes.
- Wide range of applications: Provide a powerful tool for peptide structure and function studies, drug screening, bio-imaging and other fields.
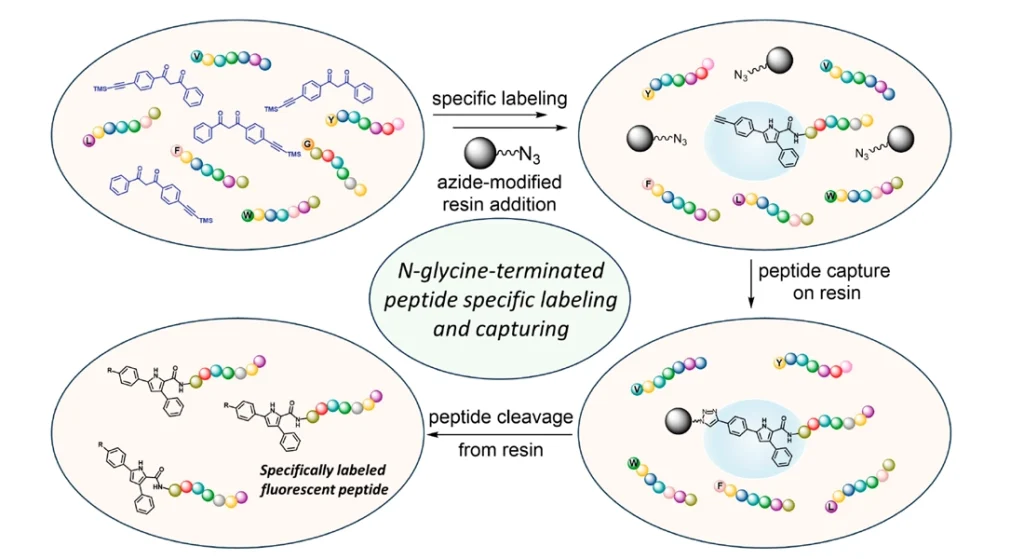
This study provides a method for selective labeling of N-terminal glycine peptides using 1,3-diphenyl 1,3-diones to provide pyrrole-based fluorescent products. It is strictly selective for N-terminal glycine due to the “dual activation” mechanism. It provides a theoretical basis for customized N-glycine-based fluorescence modification and a stability test method for rapid identification of N-terminal glycine-containing peptides.