Introduction:Welcome to the blog of MOL Changes.ltd! In this article, professional peptide MDs will give you a detailed guide on how to reconstitute Tirzepatide powder, a drug for diabetes and weight loss[1][5]. Whether you are a healthcare professional, or a patient, understanding how to properly reconstitute Tirzepatide peptide is critical to getting the most out of Tirzepatide.
What is Tirzepatide Peptide?
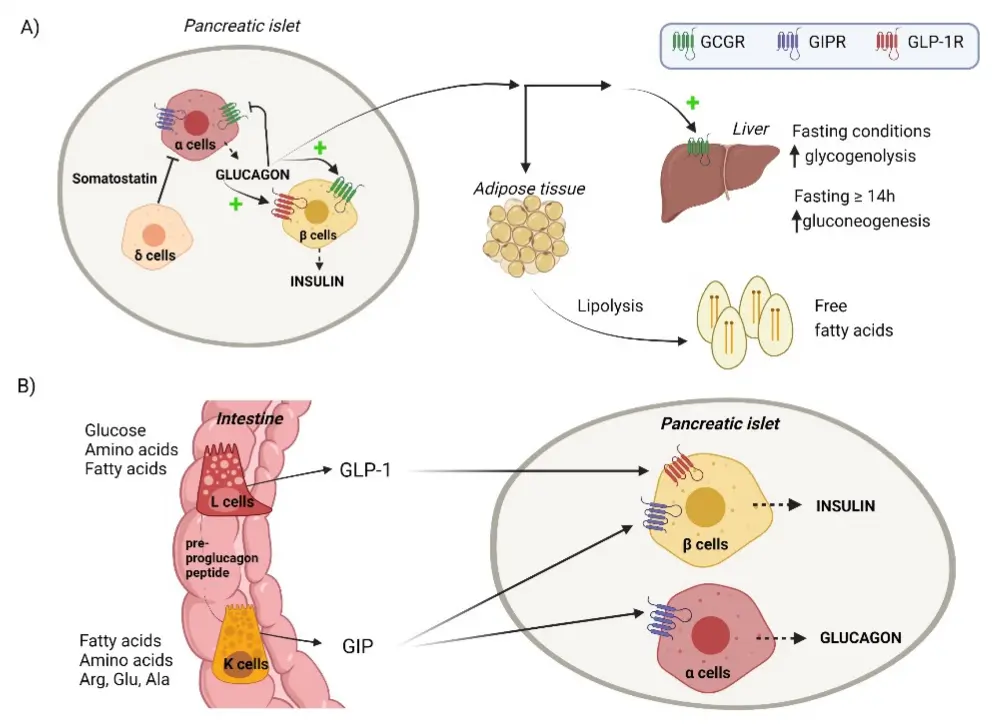
What is the mechanism of action of Tirzepatide?
Tirzepatide peptide is a dual agonist of hormone receptors known as GIP (glucose-dependent insulinotropic MOL Changes) and GLP-1 (glucagon-like peptide-1). These hormones are produced naturally in the body and they play an important role in regulating blood sugar levels and metabolism.
Stimulates insulin release: Tirzepatide activates GIP receptors and GLP-1 receptors, both of which are located on pancreatic beta cells, thereby stimulating insulin release. As a result of activation of these receptors, the pancreas releases insulin in response to high blood sugar. Insulin lowers blood sugar levels because it helps cells absorb glucose from the blood.
Inhibits the release of glucagon: Tirzepatide also inhibits the release of glucagon, another hormone produced by the pancreas. Glucagon raises blood sugar by causing the liver to release stored glucose. Tirzepatide stops excess glucose production by reducing the release of glucagon.
Appetite regulation and gastric emptying: In areas of the brain involved in appetite regulation, GLP-1 receptors are also present. Tirzepatide promotes satiety and reduces food intake by activating these receptors. In addition, GLP-1 controls the rate at which nutrients, including glucose, are absorbed into the bloodstream after a meal by reducing gastric emptying.
Weight loss: The dual agonist action of Tirzepatide at the GIP and GLP-1 receptors was associated with significant weight loss. By regulating fat utilization and increasing satiety, it may help reduce calorie intake and increase energy expenditure.
Tirzepatide works by activating the GIP and GLP-1 receptors to address various issues,including blood sugar regulation, insulin release, glucagon suppression, and appetite control.
How to Reconstitute Tirzepatide Powder?
The reconstruction of Tirzepatide powder is mainly divided into six steps.
Step 1 Find the right environment
Find a clean and well-lit area to prepare the drug while reconstituting Tirzepatide powder. Make sure the surface is free of any contamination.MOL Changes

Step 2 Prepare necessary items
Tirzepatide Powder
Sterile water for injection
Vial adapter
Syringe

Step 3 Wash your hands
Always wash your hands thoroughly with soap and water before reconstituting Tirzepatide powder to prevent the introduction of bacteria or other harmful substances

Step 4 Reconstitute Tirzepatide powder
① Check the Tirzepatide peptide vial for any signs of damage or contamination. Do not use if vial is broken or powder has changed color;
②Remove the protective cap from the vial containing Tirzepatide powder;
③ Wipe the rubber stopper on the vial with an alcohol swab for disinfection;
④ Connect the vial adapter to the vial;
⑤ According to the instructions, pick up the syringe and draw an appropriate amount of sterile water for injection;
⑥ Slowly inject sterile water into the vial of Tirzepatide peptide. Direct the stream of water toward the sides of the vial to minimize foaming.
⑦ Gently rotate the vial to mix the water and powder, do not shake vigorously;
⑧ Check the reconstituted solution for any particles or clumps, make sure the powder is completely dissolved before proceeding. If any problems are found, do not use this solution and discard it properly.

Step 5 Take out the mixed Tirzepatide solution
①Invert the vial and insert the syringe into the vial adapter;
② Slowly pull back the plunger to draw out the required dose of Tirzeparatide solution;
③ Check the syringe for air bubbles, if present, tap the syringe lightly to expel it.
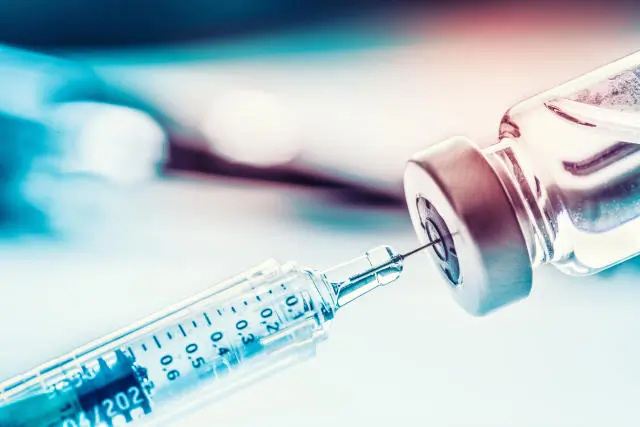
Step 6 Administration
After withdrawing the Tirzepatide solution, inject at the correct injection site as directed.
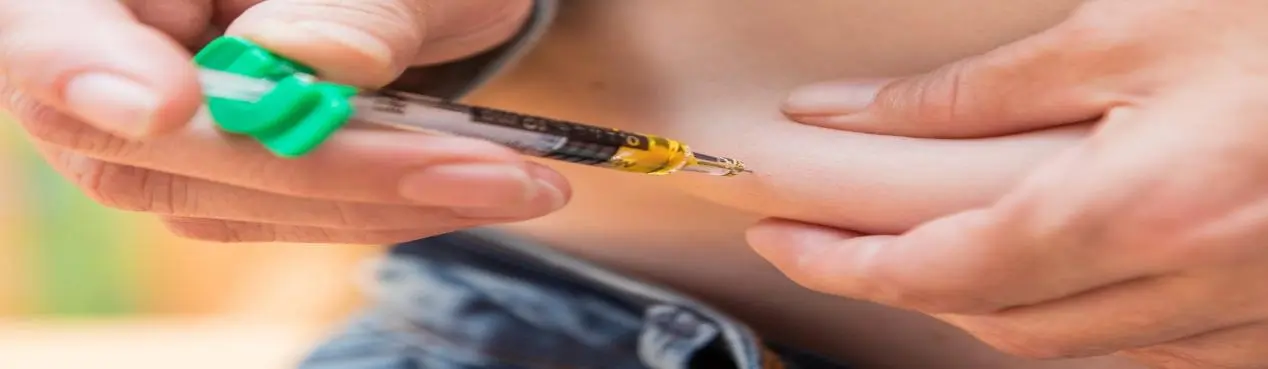
What are the benefits of Tirzepatide peptide?
- Promote weight loss[3][6]. By suppressing appetite, increasing satiety, reducing calorie intake, so as to achieve weight loss.
- Control and stabilize blood sugar levels[2][4]. Tirzepatide can control and stabilize blood sugar levels by regulating GLP-1 and GIP (to reduce glycated hemoglobin (HbA1c) levels.
- Treatment of obesity-related diseases. Tirzepatide can effectively reduce obesity-related diseases (three highs) and protect the cardiovascular system.
- Convenient. Tirzepatide only needs to be injected once a week, which greatly facilitates the lives of patients.
- The administration method is flexible. Tirzepatide can be used alone or with other diabetes medicines to work better.
Strong weight loss effect makes Tirzepatide recommended by expert consensus
Experts agree that obesity—particularly abdominal obesity—is an independent risk factor for ASCVD. Weight loss is not just about weight control, but about improving multiple ASCVD risk factors to reduce ASCVD morbidity and mortality. Antidiabetic drugs are currently classified into 4 classes based on their weight loss effects: very strong, strong, moderate and no effect. Experts agree that Tirzepatide and Semaglutide are the most effective hypoglycemic agents for weight loss. In fact, the SURPASS series of studies on Tirzepatide have demonstrated its significant effect on weight loss.
The three dose groups of Tirzepatide lyophilized powder (5 mg, 10 mg, 15 mg) showed a weight loss effect superior to all control groups (including placebo or active control drugs) in the SURPASS series of studies, with weight loss ranging from 6.5% to 13.9%. This study demonstrates that patients with T2DM can use more effective medications for clinical weight loss. These drugs are effective in reducing ASCVD risk. In addition, in the recent SURPASS-AP-Combo study mainly involving Chinese adults with T2 diabetes, the three dose groups of Tirzepatide also showed significant weight loss effects (Tirzepatide decreased by 6.5% to 9.4%; the control group increased by 2.1%) , P < 0.05), which provides evidence-based medical evidence for future use. Expert consensus believes that ASCVD is closely related to multiple metabolic indicators (blood pressure, blood lipids, blood sugar, etc.), except for weight management.
Expert consensus recommends that non-pregnant patients keep HbA1c below 7.0% to reduce the risk of ASCVD. It’s about weight loss. In the SURPASS series of studies, all three dose groups of Tirzepatide showed a significant hypoglycemic effect (attainment rate of 75% to 97%, HbA1c of 7.0%).
MOL Changes weight loss drug Vs Traditional weight loss drug
Traditional MOL Changes Efficacy
Some traditional weight loss products have little effect on weight loss (like Rimonaban);
Some stop using them will lead to weight rebound(like Sibutramine).
The effect is remarkable, and its weight loss effect can usually reach 10%-20%;
There will be basically no weight rebound after stopping use.
Side effectsPotentially lead to side effects such as cancer, cardiovascular disease, and neurological damage.As biological drugs, have relatively small side effects.Way of administrationtaken orally and taken every day.Through injection and most are once a week[7]
How to store Tirzepatide lyophilized powder?
Lyophilized powder form Tirzepatide should be stored in the refrigerator (between 2-8ºC). Keep tirzepatide in original package until use to protect from light. Single-dose pens (Tirzepatide injection liquid) can be stored at room temperature for 21 days, if needed. Do Not freeze Tirzepatide. Freezing can potentially damage the medication and affect its effectiveness. Store your medication only as directed by your Tirzepatide supplier such as MOL Changes.ltd. Make sure you understand how to store your peptide properly.
Tirzepatide powder dosage – How to use Tirzepatide?
MOL Changes.ltd as a Tirzepatide supplier. We only good at producing high quality Tirzepatide powder. The Tirzepatide powder dosage and administration information provided here is for informational purposes only and should not be considered as medical advice. The dosing of tirzepatide lyophilized powder can vary based on its intended use for type 2 diabetes and weight loss. Based on multiple studies, here is a general tirzepatide dosage guide:
Tirzepatide dosage for Type 2 Diabetes
Tirzepatide(Mounjaro) peptide is used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. The dosing typically follows this pattern:
Initial Dose: 2.5 mg subcutaneous injection once a week for 4 weeks.
Maintenance Dose: Increase to 5 mg subcutaneous injection once a week after the initial 4 weeks.
Titration: If additional glycemic control is needed, the dose can be increased in 2.5 mg increments after at least 4 weeks at the current dose.
Maximum Dose: The maximum recommended dose is 15 mg subcutaneous injection once a week.
Please note that the initial 2.5 mg dose is for treatment initiation and is not effective for glycemic control. You will need to adjust your dosage based on your response and needs.
Tirzepatide dosage for Weight Loss
Tirzepatide peptide has been shown to help lose weight in overweight patients. The dosing for weight loss may be different and can be informed by clinical trials such as SURMOUNT-1 and SURMOUNT-2. MOL Changes.ltd as Tizepatide powder manufacturer don’t teach how to use Tirzepatide. But a potential weight loss protocol could be:
Initial Dose: 2.5 mg subcutaneous injection once a week for the first four weeks.
Progressive Increase: Increase the dose to 5 mg weekly in weeks 5-8, 7.5 mg weekly in weeks 9-12, and 10 mg weekly in weeks 13-16.
Further Adjustment: Depending on individual response and tolerance, consider increasing to 12.5 mg weekly in weeks 17-20, and up to a maximum dose of 15 mg weekly from week 21 onwards.
Frequency and Duration: Administer once-weekly subcutaneous injections, and studies using this protocol have lasted 24-72 weeks.
It’s essential to consult your healthcare provider before making any changes to your medication dosage. This guide is a general overview and should not replace personalized medical advice. Your healthcare provider will determine the most appropriate dosing regimen for your specific condition and needs.MOL Changes.ltd
Frequently Asked Questions(FAQ)FAQ
1.Q: How should the reconstituted Tirzepatide powder solution be stored?
A: After reconstitution, Tirzepatide solution should be stored in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze the solution. The reconstituted solution should be used within 8 hours
2.Q: Can Tirzepatide lyophilized powder be mixed with other medicines?
A: No, Tirzepatide should not be mixed with any other medicines in the same syringe. They should be administered separately to avoid drug conflicts.
3.Q: What if I miss a dose of Tirzepatide peptide?
A: If you miss a dose of Tirzepatide take the missed dose as soon as you remember it, if within 4 days after the missed dose. However, if it has been more than 4 days since your last dose then skip the missed dose and continue your regular dosing schedule. Do not inject two doses within 3 days of each other.
4.Q: Are there any side effects of Tirzepatide?
A: As with any medicine, Tirzepatid may cause side effects. Common side effects include nausea, vomiting, diarrhea, and injection site reactions. Changes in your kidneys (such as change in the amount of urine), vision (such as decreased/blurred vision), pancreas, or gallbladder disease (such as persistent nausea/vomiting, severe stomach/abdominal pain) are serious side effects that need immediate medical attention.
5.Q: Can Tirzepatide powder be mixed ahead of time?
A: No, it is recommended to mix Tirzepatide powder immediately before use to ensure its potency.
6.Q: What should I do if I accidentally shake the vial?
A: If you accidentally shake the vial, let it sit for a few minutes to allow air bubbles to settle. Do not use if solution foams or contains visible particles.
7.Q: Can the vial adapter be reused?
A: No, the vial adapter is for single use only. Dispose of it properly after each use.
8.Q: How should Tirzepatide be administered correctly?
A: It is recommended to take once a week according to the instructions. It can be taken with meals or on an empty stomach. It can be injected into the thigh, abdomen or upper arm. The injection site should be rotated each time.
Points to Note
![]() Tirzepatide is a MOL Changes used to treat type 2 diabetes and obesity.
Tirzepatide is a MOL Changes used to treat type 2 diabetes and obesity.
![]() Proper reconsititutionof Tirzepatide is critical to its effectiveness.
Proper reconsititutionof Tirzepatide is critical to its effectiveness.
![]() Follow the step-by-step guide provided to reconstitute
Follow the step-by-step guide provided to reconstitute
![]() Store the prepared solution in the refrigerator and use within 8 hours.
Store the prepared solution in the refrigerator and use within 8 hours.
![]() Do not mix Tirzepatide with other medicines in the same syringe.
Do not mix Tirzepatide with other medicines in the same syringe.
How to buy Tirzepatide powder?
Would you like to buy the best Tirzepatide Peptides from the best Tirzepatide powder suppliers in the market? You have come to the right place!
MOL Changes.ltd as a peptide factory is the best place for you to buy peptide Tirzepatide at competitive prices from small to large quantities.
Summary
Tirzepatide is very effective in the treatment of type 2 diabetes and obesity, and learning how to mix Tirzepatide powder is an important step in managing your health effectively. By following the reconstitution steps provided in the referenced article, you can properly prepare and take this medication.
For more information:Tirzepatide
Author of this article:
Dr. Jean Zeng graduated from king’s college london Faculty of Life Sciences & Medicine.
Scientific Journal paper Author:
Université de Lorraine, CHRU de Nancy, hôpital Brabois Adultes, service d’endocrinologie, diabétologie et nutrition, rue du Morvan, 54500 Vandœuvre-lès-Nancy, France
UAB Diabetes Research Center, University of Alabama at Birmingham, Birmingham, AL, USA
3,Qi Liu
Cardiovascular Research Institute of Wuhan University, Wuhan 430060, Hubei, PR China
Department of Medicine, Koc University School of Medicine, Istanbul 34010, Turkey
5,Francine Mendoza B.S., Pharm.D. Candidate
Harold Schnitzer Diabetes Health Center (HSDHC), Oregon Health and Science University (OHSU), 3270 SW Pavilion Loop, Portland, OR 97239
In no way does this doctor/scientist endorse or advocate the purchase, sale, or use of this product for any reason. MOL Changes.ltd has no affiliation or relationship, implied or otherwise, with this physician. The purpose of citing this doctor is to acknowledge, acknowledge and commend the exhaustive research and development work done by the scientists working on this peptide.
Referenced Citations
[1]FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes
[2]The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials”
[3]”Tirzepatide significantly reduced A1C and body weight in people with type 2 diabetes in two phase 3 trials from Lilly’s SURPASS program” (Press release). Eli Lilly and Company. 17 February 2021. Archived from the original on 28 October 2021. Retrieved 28 October 2021 – via PR Newswire
[4]Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, et al. (January 2021). “Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes“. The Journal of Clinical Endocrinology and Metabolism. 106 (2): 388–396. doi:10.1210/clinem/dgaa863. PMC 7823251. PMID 33236115.
[5]Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, Rodríguez Á (February 2022). “Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial“. JAMA. 327 (6): 534–545. doi:10.1001/jama.2022.0078. PMC 8826179. PMID 35133415.
[6]”Lilly : Phase 3 Tirzepatide Results Show Superior A1C And Body Weight Reductions In Type 2 Diabetes”. Business Insider. RTTNews. 19 October 2021. Archived from the original on 28 October 2021. Retrieved 28 October 2021.
[7]Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. (4 June 2022). “Tirzepatide Once Weekly for the Treatment of Obesity“. NEJM. 387 (3): 205–216. doi:10.1056/NEJMoa2206038. PMID 35658024. S2CID 249385412. Archived from the original on 5 June 2022. Retrieved 6 June 2022.
Disclaimer: The content in this article is for general informational purposes only. It may not be accurate, complete and should not be considered medical, legal, financial or other professional advice. Any actions or decisions taken based on this information are the sole responsibility of the user