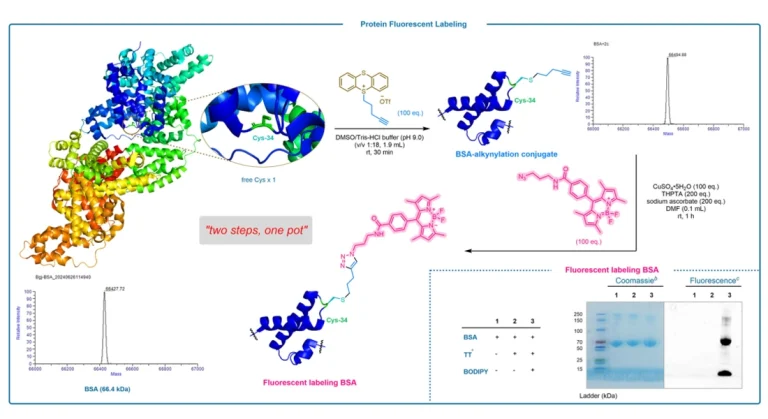
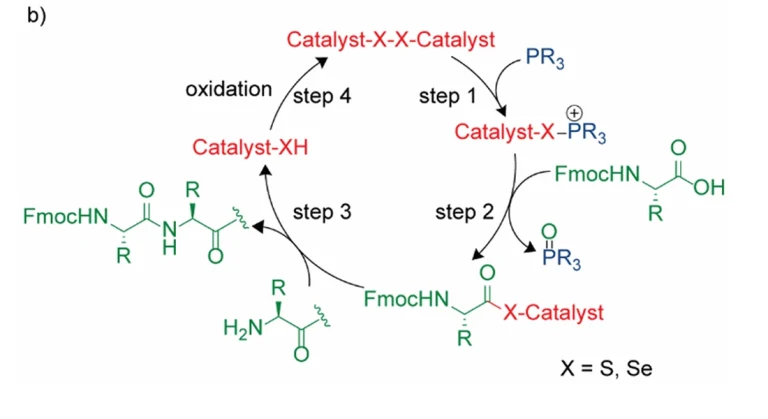
Research background and challenges
Selective ring closure of multiple sulfhydryl compounds is a key step in the synthesis of sulfur-containing heterocyclic compounds, but the traditional method has the following problems:
- Multiple sulfhydryl reaction activities are similar, making it difficult to achieve selective control. Many side reactions, complex products, and difficult to isolate and purify. The reaction conditions are harsh and the scope of application is limited. We have successfully developed a new selective oxidative ring-closing technology, which completely solves the above problems and achieves efficient and precise ring-closing reaction.
Technical breakthroughs
- Innovative oxidation system: The first alkyne amide-based oxidation system is proposed, which significantly improves the selectivity.
- Unique reaction design: through a variety of different positions of the sulfhydryl substrate, the precise ring closure of multiple sulfhydryl substrates is achieved.
Technical Advantages
- High selectivity: precise identification of target sites among multiple sulfhydryl substrates to achieve single product generation.
- High efficiency: the reaction conditions are mild, and the reaction yield is significantly improved.
- Universality: applicable to the synthesis of various sulfur-containing heterocyclic compounds.
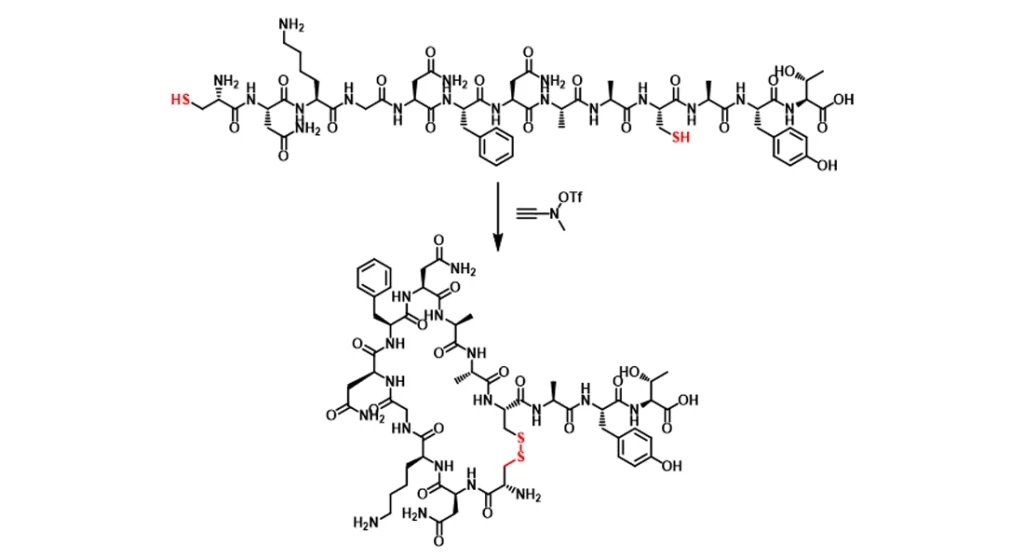
Application Fields
- Drug synthesis: construction of sulfur-containing heterocyclic drug molecules (e.g., thiophene, benzothiophene, dithiopyrrolidine, etc.).
- Functional materials: preparation of sulfur-containing polymer materials (e.g. self-repairing materials, conductive polymers, etc.).
- Chemical biology: study the formation and function of protein disulfide bonds.