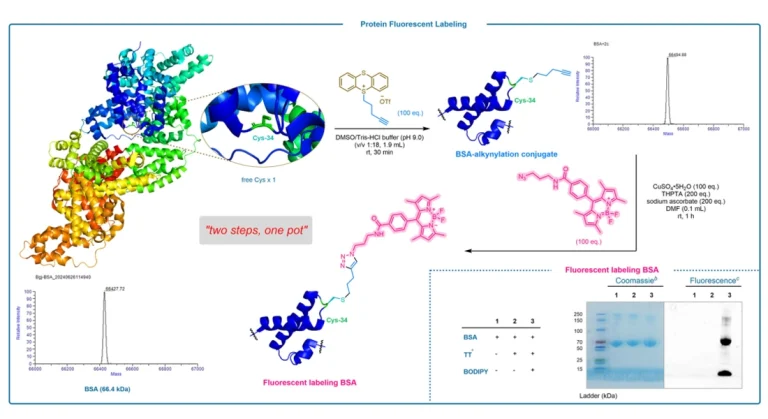
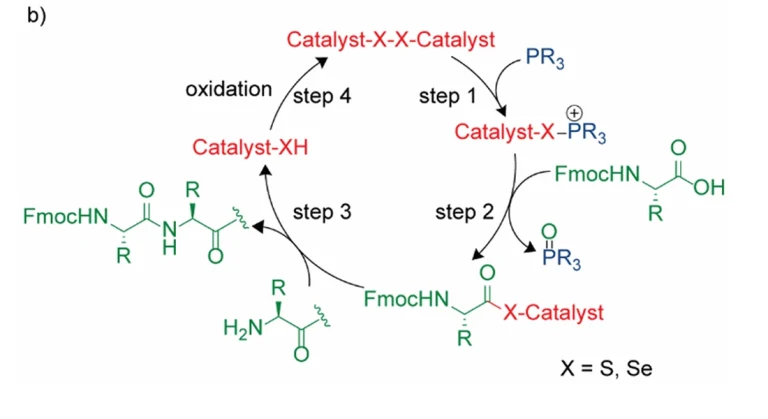
Glycosylation, as an important post-translational modification of proteins, has a significant impact on the properties of proteins such as structure, stability, antigenicity and immunogenicity. However, conventional methods for the preparation of N-glycosylated peptides usually rely on the formation of amide bonds, a process that may be interfered by acidic or basic functional groups, thus limiting their applications. To overcome this challenge, a novel strategy – a site-selective Giese addition reaction catalyzed by photoredox – was developed to successfully achieve the preparation of N-glycosylated peptides.
Technological breakthrough
A novel visible light catalyst was developed to realize the glycosylation modification of peptides under mild conditions.
Breaking through the limitations of traditional methods on glycosyl donors and amino acid residues, the precise coupling of multiple glycosyl groups and peptides was realized.
An efficient and versatile platform for photocatalytic peptide glycosylation modification has been established, providing a powerful tool for the synthesis of complex glycopeptides.
Technical Advantages
- The reaction is highly site-selective and orthogonal, capable of efficiently generating N-glycosylated peptides.
- Capable of modifying complex peptides derived from natural proteins, including biologically active peptides and peptide drugs.

The photocatalytic peptide glycosylation modification technology provides a new solution for the precise synthesis of glycopeptides, which is of great scientific significance and application value. The development of this technology will promote the progress in the fields of glycobiology, drug discovery and materials science, among others.