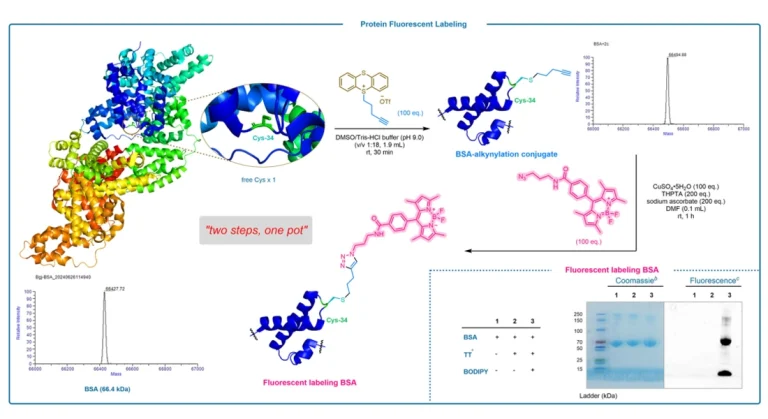
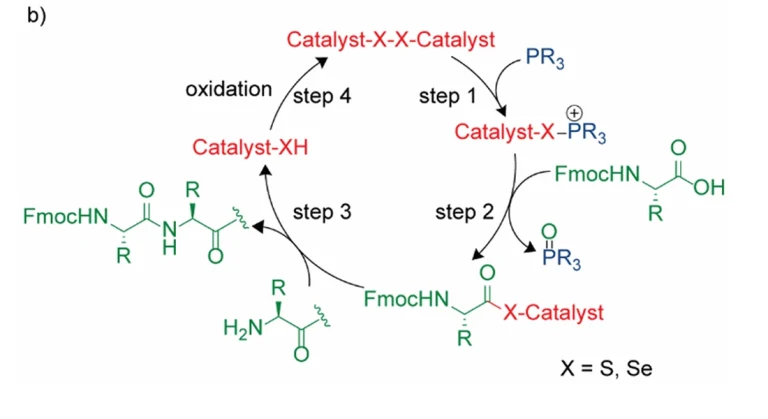
Peptide synthesis is a key technology for obtaining peptides with specific sequences and functions, which is widely used in the fields of biomedicine and material science. The traditional liquid-phase synthesis method is cumbersome and low yielding, which is difficult to meet the increasing demand, and was revolutionized by Bruce Merrifield’s Solid Phase Peptide Synthesis (SPPS) in 1963, for which he was awarded the Nobel Prize in Chemistry in 1984. However, the traditional solid phase synthesis technique requires repetitive steps of deprotection, coupling and washing to gradually extend the peptide chain, and finally cutting the target molecule from the carrier, which is not in line with the concept of green chemistry. Reverse Phase Solid Phase Peptide Synthesis (RP-SPPS) employs hydrophobic carriers (e.g., polystyrene resin) and polar solvents (e.g., DMF), which is the opposite of normal phase solid phase synthesis. In recent years, RP-SPPS has been more and more widely used in the field of peptide synthesis due to its unique advantages.
Advantages
Compared with the traditional forward solid-phase synthesis, reverse solid-phase synthesis (RP-SPPS) has its unique advantages.
Completely mimic the peptide synthesis in the organism: It is highly consistent with the direction of peptide synthesis in the organism, which provides an in vitro experimental basis for the study of peptide and protein synthesis in the organism.
Easier purification: The hydrophobic carrier is easy to be washed with organic solvents, effectively removes excessive reagents and by-products, and simplifies the purification steps.
Unique carboxy-terminal modification: RP-SPPS enables carboxy-terminal modification to be synthesized directly on the solid-phase resin, eliminating the need for pre-modification or modification after peptide chain synthesis.
More in line with green chemistry: Reversed-phase solid-phase synthesis is resistant to reactions under aqueous conditions and requires only a small amount of organic solvent to efficiently complete the coupling.
Suitable for unprotected strategies: RP-SPPS is suitable for most unprotected synthetic peptide strategies on the market, making peptide synthesis easier.

Peptide chain extension in N-C direction
Application Prospects
With the development of new carriers, coupling reagents and automated synthesizers, RP-SPPS will play a greater role in the field of peptide synthesis, providing more efficient and convenient peptide synthesis solutions for the fields of biomedicine and material science.