Peptide Modifications
peptide Phosphorylation
What is peptide Phosphorylation?
Cellular phosphorylation is a modification that can change the activity, binding properties, or cellular localization of proteins or enzymes. It is also an important regulatory process that plays a critical role in many disease pathologies, including inflammation, cancer, neurological and metabolic disorders. Enzymes that phosphorylate proteins at specific amino acid residues (e.g., tyrosine, serine, and threonine) are called kinases. Enzymes that remove a phosphate group by hydrolysis are termed phosphatases. Because phosphorylation is central to most signal transduction pathways, phosphorylated peptides (i.e., phosphopeptides) are used for the analysis of protein kinases and phosphatases in may cellular assays.
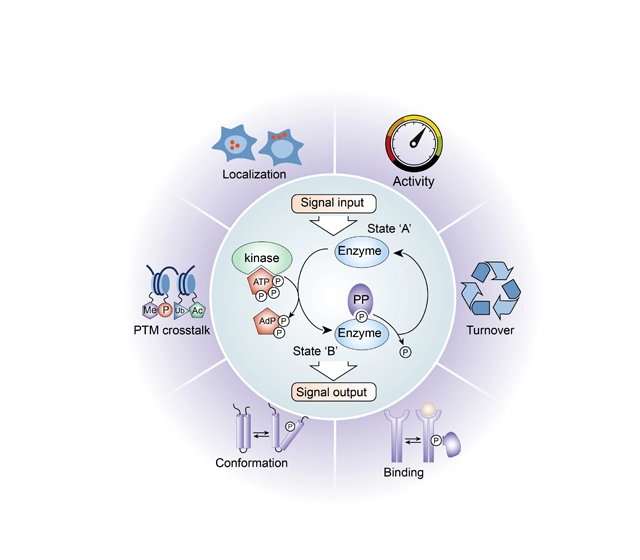
Typical location of phosphoryl groups
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation. CPC Scientific can synthesize phosphopeptides and incorporate single or multiple combinations of phosphoserine (pS), phosphothreonine (pT), or phosphotyrosine (pY).
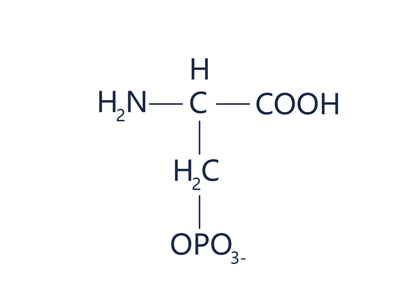
Phosphoserine
Phosphoserine is a short peptide or peptide chain containing a phosphorylated serine (pSer), i.e., a peptide fragment in which the hydroxyl group (-OH) of a serine residue is modified by a phosphate group (-PO₄²-).
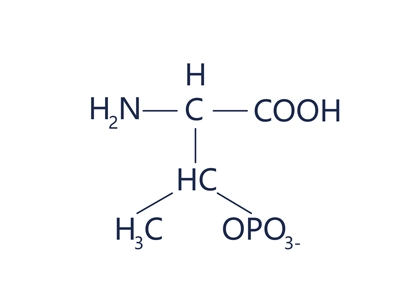
Phosphothreonine
Phosphothreonine is a short peptide or polypeptide chain containing phosphothreonine (pThr), i.e. a peptide in which the hydroxyl group (-OH) of a threonine residue is modified by a phosphate group (-PO₄²-).
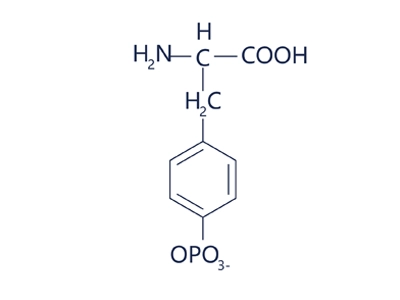
Phosphotyrosine
Phosphotyrosine is a short peptide or polypeptide chain containing phosphotyrosine (pTyr), i.e. a peptide in which the phenolic hydroxyl group (-OH) of a tyrosine (Tyr) residue is modified by a phosphate group (-PO₄²-).
Featured Citations
PRMT5 C-terminal Phosphorylation Modulates a 14-3-3/PDZ Interaction Switch*
PRMT5 is the primary enzyme responsible for the deposition of the symmetric dimethylarginine in mammalian cells. In an effort to understand how PRMT5 is regulated, we identified a threonine phosphorylation site within a C-terminal tail motif, which is targeted by the Akt/serum- and glucocorticoid-inducible kinases. While investigating the function of this posttranslational modification, we serendipitously discovered that its free C-terminal tail binds PDZ domains (when unphosphorylated) and 14-3-3 proteins (when phosphorylated). In essence, a phosphorylation event within the last few residues of the C-terminal tail generates a posttranslational modification-dependent PDZ/14-3-3 interaction “switch.” The C-terminal motif of PRMT5 is required for plasma membrane association, and loss of this switching capacity is not compatible with life. This signaling phenomenon was recently reported for the HPV E6 oncoprotein but has not yet been observed for mammalian proteins. To investigate the prevalence of PDZ/14-3-3 switching in signal transduction, we built a protein domain microarray that harbors PDZ domains and 14-3-3 proteins. We have used this microarray to interrogate the C-terminal tails of a small group of candidate proteins and identified ERBB4, PGHS2, and IRK1 (as well as E6 and PRMT5) as conforming to this signaling mode, suggesting that PDZ/14-3-3 switching may be a broad biological paradigm.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.