Peptide Modifications
N-terminal modification
What is N-terminal modification?
N-terminal acetylation removes the charge from the amino terminus of a peptide. In general, acetylation modification is recommended if the peptide is to mimic its natural structure in the protein. In addition, the acetylation modification stabilises the peptide chain and makes it less susceptible to enzymatic degradation at the end of the peptide chain.
In addition to acylation, other N-terminal modifications (e.g., urea, carbamate, sulfonamide, alkylamine) can be performed.
Radioligands are often attached to the N-terminus of a peptide if the pharmacodynamics of the peptide allow it. For example, DOTA, NOTA, NODAGA, etc. can be attached.
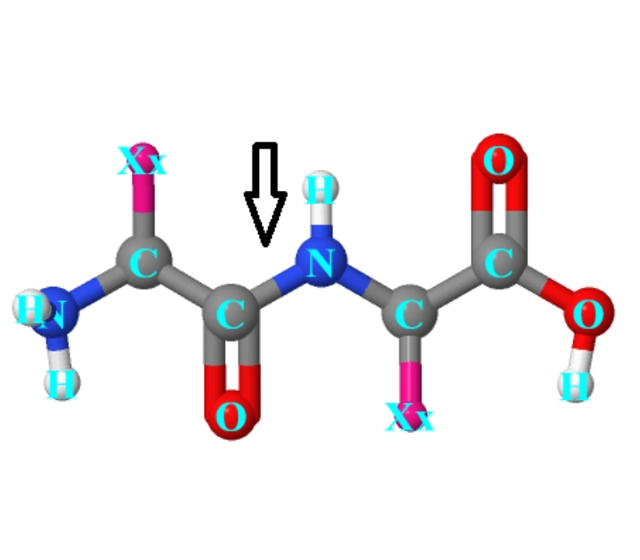
N-terminal modification technical
Modification type
N-terminal modification improves the stability of peptides because this modification generates a structure similar to the natural protein structure. Thus, this modification method can improve the biological activity of the polypeptide and prevent enzymatic degradation.
5-FAM |
BSA (-NH2 of N terminal) |
Hexanoic acid |
|---|---|---|
|
5-FAM-Ahx |
CBZ |
HYNIC |
|
Abz |
Dansyl |
KLH (-NH2 of N terminal) |
|
Acetylation |
Dansyl-Ahx |
Lauric acid |
|
Acryl |
Decanoic acid |
Lipoic acid |
|
Alloc |
DTPA |
Maleimide |
|
Benzoyl |
Fatty Acid |
MCA |
|
Biotin |
FITC |
Myristoyl |
|
Biotin-Ahx |
FITC-Ahx |
Octanoic acid |
|
BOC |
Fmoc |
OVA (-NH2 of N terminal) |
|
Br-Ac- |
Formylation |
Palmitoyl |
Featured Citations
Proteome-derived peptide libraries for deep specificity profiling of N-terminal modification reagents
Protein and peptide N termini are important targets for selective modification with chemoproteomics reagents and bioconjugation tools. The N-terminal α-amine occurs only once in each polypeptide chain, making it an attractive target for protein bioconjugation. In cells, new N termini can be generated by proteolytic cleavage and captured by N-terminal modification reagents that enable proteome-wide identification of protease substrates with tandem mass spectrometry (LC-MS/MS). An understanding of the N-terminal sequence specificity of the modification reagents is critical for each of these applications. Proteome-derived peptide libraries in combination with LC-MS/MS are powerful tools for profiling the sequence specificity of N-terminal modification reagents. These libraries are highly diverse, and LC-MS/MS enables analysis of the modification efficiencies of tens of thousands of sequences in a single experiment. Proteome-derived peptide libraries are a powerful tool for profiling the sequence specificities of enzymatic and chemical peptide labeling reagents. Subtiligase and 2-pyridinecarboxaldehyde (2PCA) are two reagents that have been developed for selective N-terminal peptide modification and can be studied using proteome derived peptide libraries. This protocol outlines the steps for generating N-terminally diverse proteome-derived peptide libraries and for applying these libraries to profile the specificity of N-terminal modification reagents. We detail the steps for profiling the specificity of 2-pyridinecarboxaldehyde, a chemical modification reagent, and subtiligase, an enzymatic modification reagent. These protocols can easily be adapted to alternative proteome sources and other N-terminal peptide labeling reagents.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.