Peptide Modifications
FRET Peptide
What is FRET peptide?
FRET peptide labelling has a donor molecule and an acceptor (bursting agent) molecule. In most cases, the donor and acceptor pairs are two different dyes. If the acceptor is a non-fluorescent dye (bursting agent), the transferred energy from the fluorescent donor is converted into molecular vibrations. When FRET is terminated (by separating the donor and acceptor), an increase in donor fluorescence can be detected. When both donor and acceptor dyes are fluorescent, the transferred energy is emitted as longer wavelength light, allowing changes in the donor and acceptor fluorescence intensity ratio to be measured. For efficient FRET bursting, the fluorophore and bursting agent molecules must be in close proximity to each other (~10-100 Å) and the absorption spectrum of the bursting agent must overlap with the emission spectrum of the fluorophore. Careful comparison of the fluorescence spectrum of the donor and the absorption spectrum of the bursting agent is required when designing donor-bursting agent FRET systems.
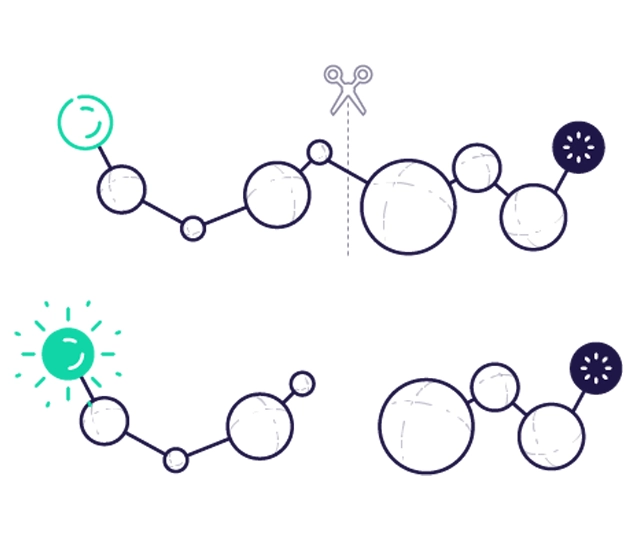
FRET peptides science
Key benefits of FRET peptides:
·Real-time monitoring of enzymatic reactions and protein activity.
·Extremely high detection sensitivity due to unique proximity-based energy transfer.
·Versatility for in vitro and in vivo applications, providing insight into complex biological processes.
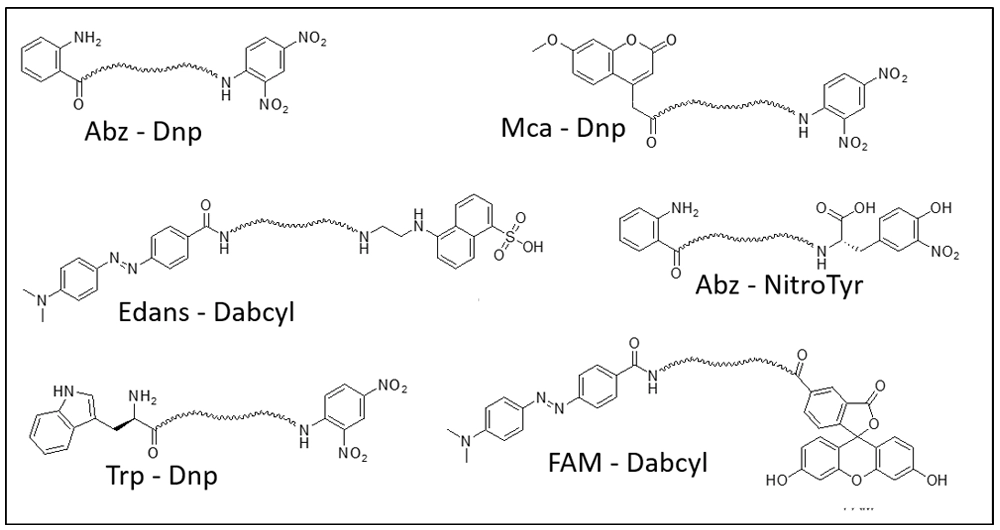
Fluorophore |
Quencher |
Donor Ex/Em |
|---|---|---|
|
Abz |
Dnp 2,4-dinitrophenyl |
320 nm/420 nm |
|
Abz |
Dnp 2,4-dinitrophenyl |
320 nm/420 nm |
|
Mca |
Dnp 2,4-dinitrophenyl |
325 nm/392 nm |
|
Trp |
Dnp 2,4-dinitrophenyl |
280 nm/360 nm |
|
FAM |
Dabcyl4-([4'-dimethylamino)phenyl]azo)benzoyl |
492 nm/517 nm |
|
Lucifer Yellow |
Dabsyl4(Dimethylamino)azobenzene-4'-sulfonyl |
430nm/520nm |
Featured Citations
Ultrasensitive tumour-penetrating nanosensors of protease activity
The ability to identify cancer lesions with endogenous biomarkers is currently limited to tumours ~1 cm in diameter. We recently reported an exogenously administered tumour-penetrating nanosensor that sheds, in response to tumour-specific proteases, peptide fragments that can then be detected in the urine. Here, we report the optimization, informed by a pharmacokinetic mathematical model, of the surface presentation of the peptide substrates to both enhance on-target protease cleavage and minimize off-target cleavage, and of the functionalization of the nanosensors with tumour-penetrating ligands that engage active trafficking pathways to increase activation in the tumour microenvironment. The resulting nanosensor discriminated sub-5 mm lesions in human epithelial tumours and detected nodules with median diameters smaller than 2 mm in an orthotopic model of ovarian cancer. We also demonstrate enhanced receptor-dependent specificity of signal generation in the urine in an immunocompetent model of colorectal liver metastases, and in situ activation of the nanosensors in human tumour microarrays when re-engineered as fluorogenic zymography probes.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.