Peptide Modifications
Click Chemistry Peptides
What is Click Chemistry Peptides?
Click peptides are peptides synthesised using click chemistry. This technology is characterised by high yield, good selectivity, carbon hetero bond binding, wide applicability and easy operation. Click reaction is a key step in peptide chemistry for peptide synthesis and chemoselective modification. Click reactions have a wide range of applications, including ligation, cyclisation, biocoupling and radiolabelling.
The most common functional groups in click chemistry are azides and alkynes. Azide-alkyne cycloaddition reactions are the most common click reactions. Click chemistry has a wide range of applications in chemical synthesis, materials science, chemical biology and drug development.
Peptide click chemistry is also used for biomolecular labelling, highly selective covalent attachment to target sites, and biocoupling in proteomics studies. With the development of peptide drugs in the scientific and clinical fields, click peptides are being prepared at lower cost and with higher stability.
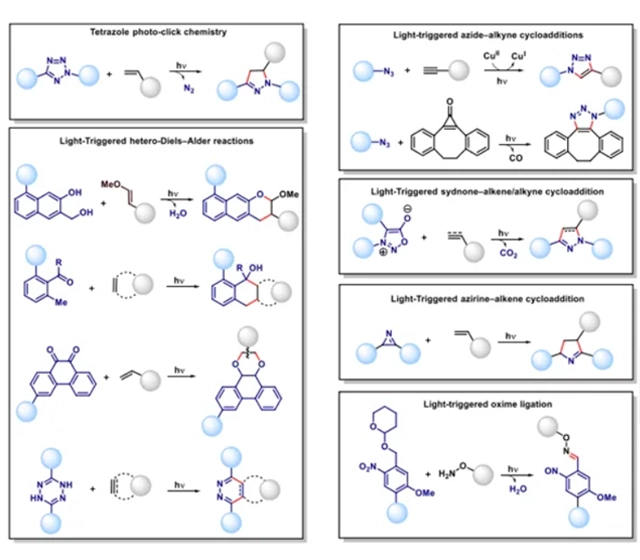
Click Chemistry Peptides typology
CuAAC and copper-free click chemistry
CuAAC (copper-catalysed azide-alkyne cycloaddition) click reactions proceed by clicking on alkyne-modified peptides and azide-modified molecules starting from the triazole bond. Copper-free click chemistry is an alternative technique to click chemistry that has a lower activation energy barrier and does not require the cytotoxic leap of a metal catalyst.
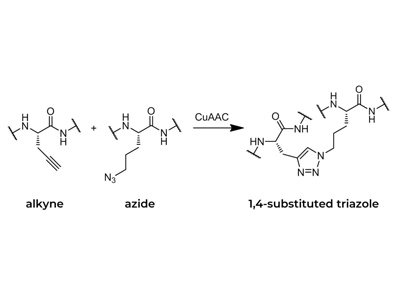
The CuAAC reaction involves the formation of rigid five-membered heterocyclic 1,2,3-triazoles under mild conditions. As far as functionality is concerned, azides are easy to introduce, stable under aqueous and oxidising conditions and highly reactive. Such triazoles are electronic equivalents of peptide bonds and have similar planarity to the amide portion, but are not susceptible to hydrolytic breakage.
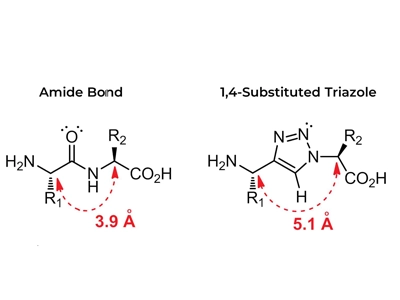
Properties such as high stability, similarity to amide bonds, and potential biological activity make the triazole bond not only a benign and easily synthesised linker, but also an important component of click chemistry.
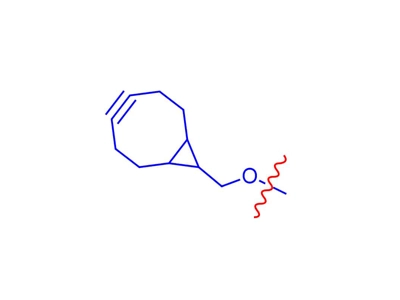
BCN Linker
BCN groups can react with azide-labelled biomolecules without the need for a copper catalyst.BCN linkers can be attached to a variety of functional groups such as amine groups, amino groups, aminooxy groups, acid groups, NHS ester groups and so on.
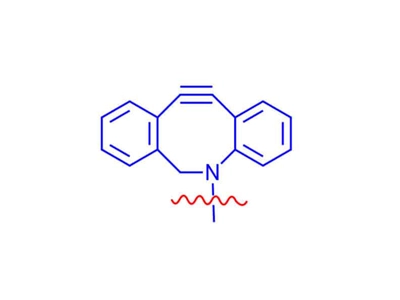
DBCO Linker
DBCO linkers can react with azide-modified biomolecules without the need for toxic Cu catalysts, making DBCO reagents ideal for use in living cells and organisms.
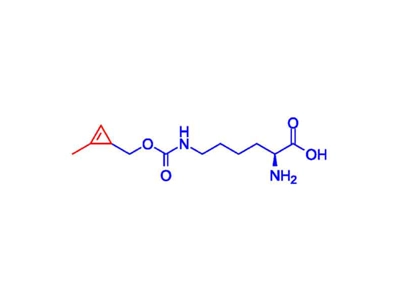
Cyclopropene Linker
Cyclopropene is the smallest unsaturated carbocyclic compound with high addition, cycloaddition, and ring opening reactivity.
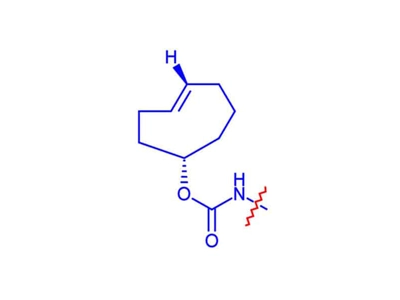
TCO Linker
TCO linkers are biological orthogonal agents of tetrazines. The cycloaddition reaction between tetrazine and trans-cyclooctene (TCO) is widely used in bioconjugation, molecular imaging and cellular diagnostics.
Tetrazine Linkers
The tetrazine linker is a bioorthogonal ligand for TCO and cyclopropene. Tetrazine linkers are chemoselective and react specifically with trans-cyclooctene (TCO). They are ideal tools for biocoupling, cell engineering and site/target specific coupling studies.
Featured Citations
Modification of DNA Nanostructures with Functional Peptides Through Copper-Free Click Chemistry
The provided protocol outlines a method for synthesizing a peptide-modified three-arm DNA nanostructure. The protocol details the functionalization of three partially complementary amino-modified single-stranded DNA molecules with azide-modified peptide, denoted "PeB," using copper-free click chemistry. This PeB peptide, derived from the neutralizing antibody HC19, effectively targets the influenza A virus. Subsequently, the peptide-functionalized DNA structures are assembled into a three-arm DNA structure, enabling multivalent presentation of the peptides to enhance binding and inhibit viral particles. While this protocol specifically describes the synthesis of simple trivalent DNA-peptide structures, the general approach can be scaled to decorate more complex DNA nanostructures such as those formed from tile- or origami-based architectures.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.