Peptide Modifications
Cyclic Peptide
What is Cyclic Peptide?
Peptide cyclization to form cyclic peptides or cyclized peptides is a frequently used strategy for the development of peptides with enhanced conformational stability compared to their linear analogs. Cyclic peptides have proven to be useful for several applications, including the mimicry of protein secondary structures, such as protein loops, and the optimization of peptide ligands with increased binding potency, selectivity, and enhanced protease stability.
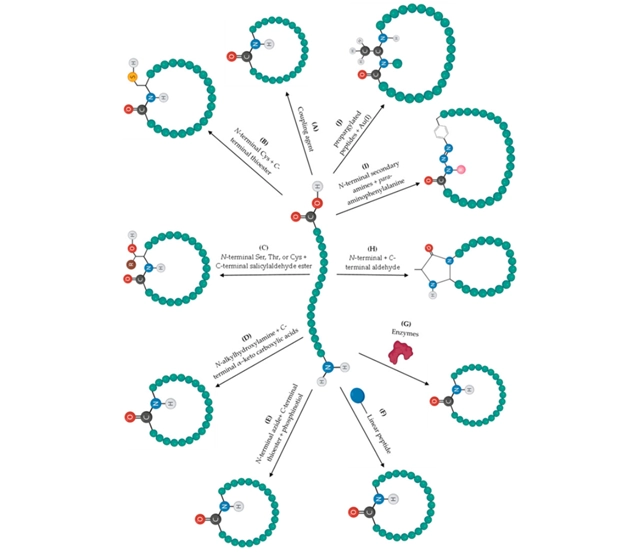
Cyclization configurations
The primary ligation approaches to synthesize lactam cyclic peptides are head-to-tail, side-chain-to-side-chain, head-to-side-chain, and side-chain-to-tail,depending on the location of the functional groups within the peptide sequence.
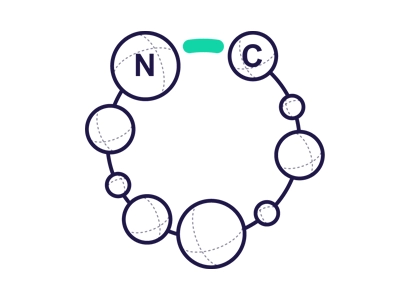
Head to Tail
·Occurs between N-terminus to C-terminus.
·Via Amide bond formation (lactamization).
·Most frequently used cyclization conferring greater stability.
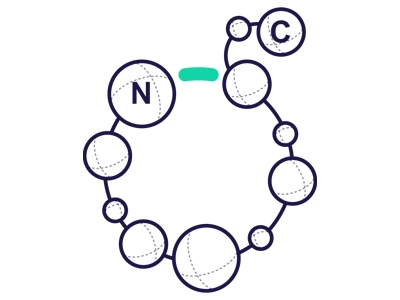
Side Chain to Head
Occurs between N-terminus and an internal COOH
(e.g. the ß-COOH-group of Asp or γ-COOH-group of Glu).
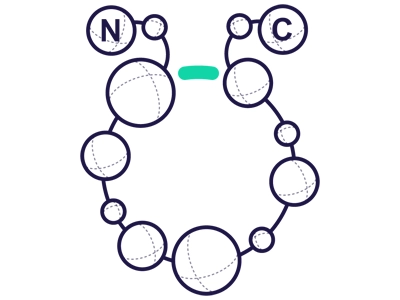
Side Chain to tail
Occurs between internal NH2s and C-terminus
(e.g. the ε-NH2–group of Lys).
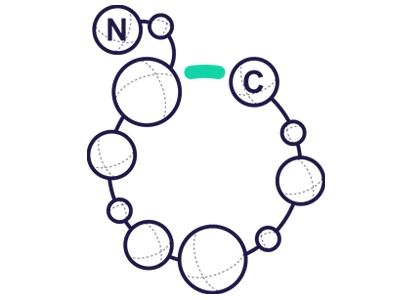
Side Chain to Side Chain
Occurs between Side chain of Lysine (ε-NH2–group of Lys) to Aspartic or Glutamic acid (γ-COOH-group).
Featured Citations
Design and NMR Studies of Cyclic Peptides Targeting the N-Terminal Domain of the Protein Tyrosine Phosphatase YopH
We report on the design and evaluation of novel cyclic peptides targeting the N-terminal domain of the protein tyrosine phosphatase YopH from Yersinia. Cyclic peptides have been designed based on a short sequence from the protein SKAP-HOM [DE(pY)DDPF (pY = phosphotyrosine)], and they all contain the motif DEZXDPfK (where Z is a phosphotyrosine or a non-hydrolyzable phosphotyrosine mimetic, X is an aspartic acid or a leucine and f is a d-phenylalanine). These peptides present a ‘head to tail’ architecture, enabling cyclization through formation of an amide bond in between the side chains of the first aspartic acid and the lysine residues. Chemical shift perturbation studies have been carried out to monitor the binding of these peptides to the N-terminal domain of YopH. Peptides containing a phosphotyrosine moiety exhibit binding affinities in the low micromolar range; substitution of the phosphotyrosine with one of its non-hydrolyzable derivatives dramatically reduces the binding affinities. These preliminary studies may pave the way for the discovery of more potent and selective peptidebased ligands of the YopH N-terminal domain which could be further investigated for their ability to inhibit Yersiniae infections.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.