Peptide Modifications
Peptide Biotinylation
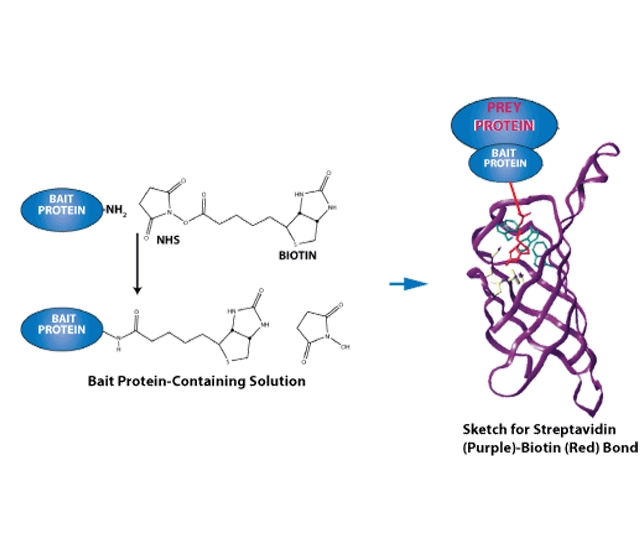
What is Peptide Biotinylation?
The detection of suitably tagged peptides or biotinylated peptides (e.g. with labeled antibodies)The separation of biotinylated peptides or tagged peptides from untagged ones (e.g. with tethered antibodies)
The tags can be small organic molecules like biotin (which binds strongly and non-covalently to streptavidin) or a short peptide sequence. The most prominent examples for peptide sequences used as tags are epitope tags like the Flag tag, the HA tag and the Myc tag. A recently developed tag is the ALFA peptide tag. For all tags, antibodies are commercially available. See below for respective sequences.
MOL Changes routinely synthesizes tagged peptides carrying various tags. The tags are usually attached at the N-terminus or the C-terminus (via lysine or cysteine), but in principle can be positioned anywhere. All tags can be separated from the peptide by a variety of different so called linkers or spacer molecules of varying length and polarity. If desired, linkers can be made cleavable, e.g. by reduction of sensitive disulfide bonds. Have a look at a list of available linker / spacer / PEGylations. Here is a representation of frequently incorporated tags.
Biotinylated and Tagged Peptides
Affinity tags such as biotin can be used for
·The detection of suitably tagged peptides or biotinylated peptides (e.g. with labeled antibodies)
·The separation of biotinylated peptides or tagged peptides from untagged ones (e.g. with tethered antibodies)
The tags can be small organic molecules like biotin (which binds strongly and non-covalently to streptavidin) or a short peptide sequence. The most prominent examples for peptide sequences used as tags are epitope tags like the Flag tag, the HA tag and the Myc tag. A recently developed tag is the ALFA peptide tag. For all tags, antibodies are commercially available. See below for respective sequences.
MOL Changes routinely synthesizes tagged peptides carrying various tags. The tags are usually attached at the N-terminus or the C-terminus (via lysine or cysteine), but in principle can be positioned anywhere. All tags can be separated from the peptide by a variety of different so called linkers or spacer molecules of varying length and polarity. If desired, linkers can be made cleavable, e.g. by reduction of sensitive disulfide bonds.
Affinity Tag |
Sequence |
Comment |
|---|---|---|
|
Flag tag |
DYKDDDDK |
Anti flag antibody |
|
HA tag |
YPYDVPDYA |
Anti HA antibody |
|
Myc tag |
EQKLISEEDL |
Anti Myc antibody |
CPP |
Sequence |
Comment |
|---|---|---|
|
Tat (47-57) |
YGRKKRRQRRR |
Cell-penetrating peptide |
|
Oligo-Arginine |
RRRRRRRRR |
Cell-penetrating peptide |
MOL Changes is also able to provide you with a wide range of reactive labels attached to peptides. Examples are the introduction of
- Azides (reaction with alkines – click chemistry)
- Maleimides (reaction with thiols, e.g. Cys)
- Thiols (reaction with maleimides)
- Cys(Pys/Npys) (reaction with thiols to form disulfides)
Featured Citations
Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2
CD33 is a myeloid specific member of the sialic acid-binding receptor family and is expressed highly on myeloid progenitor cells but at much lower levels in differentiated cells. Human CD33 has two tyrosine residues in its cytoplasmic domain (Y340 and Y358). When phosphorylated, these tyrosines could function as docking sites for the phosphatases, SHP-1 and/or SHP-2, enabling CD33 to function as an inhibitory receptor. Here we demonstrate that CD33 is tyrosine phosphorylated in the presence of the phosphatase inhibitor, pervanadate, and recruits SHP-1 and SHP-2. Co-expression studies suggest that the Src-family kinase Lck is effective at phosphorylating Y340, but not Y358, suggesting that these residues may function in the selective recruitment of adapter molecules and have distinct functions. Further support for overlapping, but nonredundant, roles for Y340 and Y358 comes from peptide-binding studies that revealed the recruitment of both SHP-1 and SHP-2 to Y340 but only SHP-2 to Y358. Analysis using mutants of SHP-1 demonstrated that binding Y340 of CD33 was primarily to the amino Src homology-2 domain of SHP-1. The potential of CD33 to function as an inhibitory receptor was demonstrated by its ability to down-regulate CD64-induced calcium mobilization in U937. The dependence of this inhibition on SHP-1 was demonstrated by blocking CD33-mediated effects with dominant negative SHP-1. This result implies that CD33 is an inhibitory receptor and also that SHP-1 phosphatase has a significant role in mediating CD33 function. Further studies are essential to identify the receptor(s) that CD33 inhibits in vivo and its function in myeloid lineage development.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.