Peptide Modifications
Chelating Peptides
What is Chelating Peptides?
Coupling peptides to metal chelators (e.g. DOTA, NOTA, etc.) offers an attractive avenue for cancer tissue imaging or therapies that transport oncotoxins to cancer cells. The interleukin 13 signalling molecule alpha 2 (IL13RA2) is a signalling molecule that is overexpressed in most malignant gliomas (GBMs) but is absent in normal cells. Targeting ligands that bind such a signalling molecule may provide a new avenue for the treatment of malignant gliomas (GBMs). Because IL13RA2 is intrinsically bound to IL13, this signalling molecule is an attractive target in therapies that translocate cancer cytotoxic molecules to malignant glioma (GBMs) cells.Peptide-1 linear (Pep-1L) has been evaluated for its ability to bind IL13RA2 in a targeted manner, and through chelating modifications, the Peptide-1 linear (Pep-1L) can transport the cytotoxin Actinium-225, Ac-225, onto malignant glioma (GBMs) cells.
Chelating Peptides service
Common List
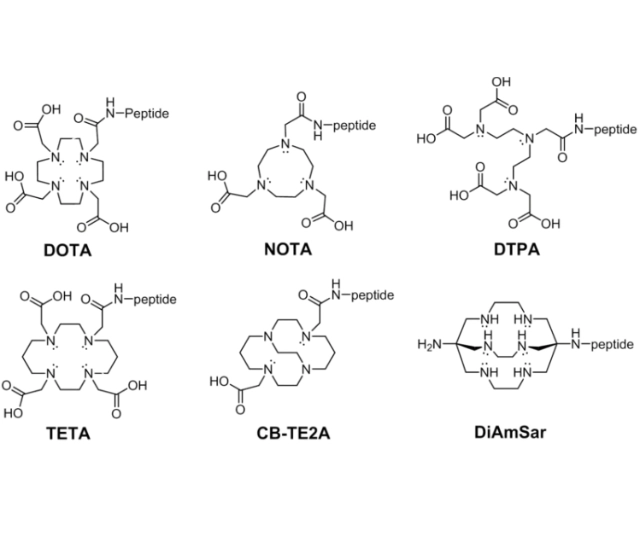
The expression or overexpression of specific signalling molecules by cancer cells has led to the development of peptide synthesis that can target these signalling molecules, and MOL Changes offers a wide range of peptide chelator modifications, including DOTA and DTPA.
abridge |
Full Name |
|---|---|
|
DOTA |
1,4,7,10-tetraazacyclododecane-N,N,N,N-tetraacetic acid |
|
NOTA |
1,4,7-triazacyclononane-N,N,N-triacetic acid |
|
TETA |
1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid |
|
DTPA |
diethylenetriaminepentaacetic acid |
|
IDA |
Iminodiacetic acid |
|
CB-TE2A |
4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane |
|
DiAmSar |
1,8-Diamino-3,6,10,13,16,19-hexaazabicyclo[6,6,6]-eicosane |
Featured Citations
New Insights in the Design of Bioactive Peptides and Chelating Agents for Imaging and Therapy in Oncology
Many synthetic peptides have been developed for diagnosis and therapy of human cancers based on their ability to target specific receptors on cancer cell surface or to penetrate the cell membrane. Chemical modifications of amino acid chains have significantly improved the biological activity, the stability and efficacy of peptide analogues currently employed as anticancer drugs or as molecular imaging tracers. The stability of somatostatin, integrins and bombesin analogues in the human body have been significantly increased by cyclization and/or insertion of non-natural amino acids in the peptide sequences. Moreover, the overall pharmacokinetic properties of such analogues and others (including cholecystokinin, vasoactive intestinal peptide and neurotensin analogues) have been improved by PEGylation and glycosylation. Furthermore, conjugation of those peptide analogues to new linkers and bifunctional chelators (such as AAZTA, TETA, TRAP, NOPO etc.), produced radiolabeled moieties with increased half life and higher binding affinity to the cognate receptors. This review describes the most important and recent chemical modifications introduced in the amino acid sequences as well as linkers and new bifunctional chelators which have significantly improved the specificity and sensitivity of peptides used in oncologic diagnosis and therapy.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.