Lyophilized Peptides
For long-term storage of peptides, we recommend storing them as a solid powder. Lyophilized peptides can be stored at -20°C or lower with minimal degradation. Customers are advised to re-inspect customized peptides every 2 years from the date of quality control release.
Peptides in solution
Peptides in solution are less stable and prone to degradation. Aqueous solutions used to dissolve peptides should be sterile and pure.
In solution, a certain amount of peptide may be degraded, depending on the amino acids present in the sequence. Some examples are listed below:
- Peptides containing methionine, cysteine or tryptophan residues should have limited storage time in solution due to oxidation. These peptides should be dissolved in an oxygen-free solvent. -
Glutamine and asparagine can be deamidated to glutamic acid and aspartic acid, respectively. -
Cysteine can undergo oxidative cyclization to form a cysteine-cysteine disulfide bond (which can be formed within or between disulfide bonds). -
The charged residues (aspartic acid, glutamic acid, lysine, arginine, histidine) are hygroscopic (absorbing water from the air) and tend to form a viscous, clear oil. This physical change may not affect the nature of the peptide. It is recommended that such peptides be lyophilized to remove trace amounts of water, then degassed with nitrogen to ensure that there is no water vapor in the vial, and then quickly capped.
To prevent damage from repeated freezing and thawing, we recommend that users only dissolve the amount of peptide required for the current experiment. Excess solution should be stored at ≥ -20 o C until needed.
Avoid moisture.
Since moisture significantly reduces the long-term stability of peptides, we recommend equilibrating the peptides in a desiccator to room temperature before opening the vials. Upon completion of peptide dispensing, the remaining peptides in the tube should be gently purged with anhydrous nitrogen, then the container should be capped, sealed with a sealing film, and stored at -20°C.
The solubility characteristics of different peptides vary considerably. Residues such as alanine (Ala), cysteine (Cys), isoleucine (Ile), leucine (Leu), methionine (Met), phenylalanine (Phe), and valine (Val) add to the peptide's hydrophobicity and solubility in aqueous solutions, and it is recommended that the customer follow these guidelines when customizing peptides.
Solubility Properties
The solubility of peptides is highly dependent on the amino acid sequence. Naturally hydrophobic peptides (A, F, G, V, L, I, M, W, P with higher propensity) require organic solvents for solubilization. Acidic peptides (higher propensity for D, E in the peptide sequence) require basic aqueous buffers for solubilization, while basic peptides (higher propensity for K, H, and R) require acidic aqueous buffers for solubilization.
Solvent Selection
Given the limitations of your assay, we recommend using the following guidelines to determine the best solvent for solubilizing peptides
Hydrophobic Peptides
To re-solubilize hydrophobic peptides, add 100 µL of DMSO and sonicate until a homogeneous solution is formed. Next, add the buffer of your choice to make a 1 mg/mL solution (the higher the peptide concentration, the greater the amount of DMSO required).
Hydrophilic (Acidic) Peptides
To re-solubilize acidic peptides, add 100 µL of 1% NH 4 OH to 1 mg of peptide and vortex. Once the solution is clear, add the buffer of your choice to make a 1 mg/mL solution.
Hydrophilic (Basic) Peptides
To reconstitute a basic peptide, add distilled water to 1 mg of peptide and vortex.
The industry standard is to deliver peptides in a lyophilized form as a Gross weight. Gross peptide weight is the total weight of all components present in the lyophilized powder. This includes the peptide of interest, any peptide impurities, water, residual solvents, and counterion.
On the contrary Net peptide weight, calculated from the Net peptide content, is the weight of only the peptide component present in the lyophilized powder. This offers more accurate concentrations of your subsequent peptide solutions. Hence, we recommended net peptide weight amounts for Custom Peptide orders.
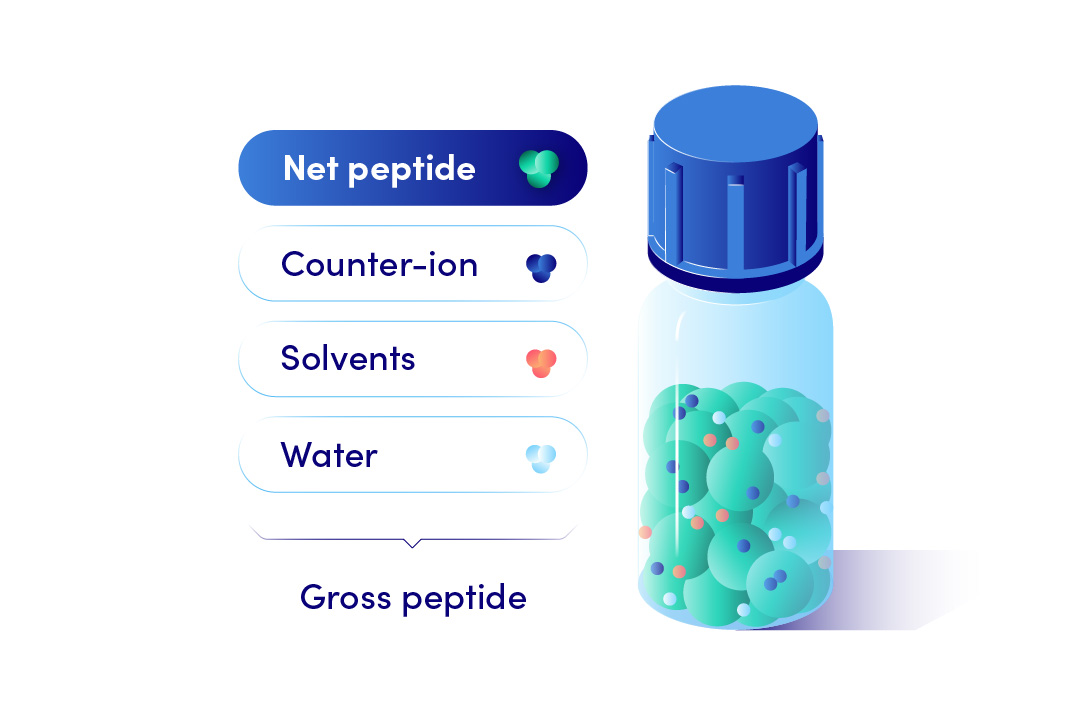
Net Peptide Content measurement is performed by amino acid analysis (AAA; limited accuracy but requires a low material amount) or elemental analysis (CHN; requires milligrams of peptide but is more accurate). Both methods yield a percentage value (ie., 75% net peptide content).
The Net Peptide Content depends on the amount of counterion present*, which is dependent on the peptide sequence. Essentially, the higher the peptide charge, the lower the peptide content
*Note: The element used to calculate net peptide content is based on the peptide counter-ion. This is done to ensure that the counter-ion does not contribute to the peptide content amount. For example, when TFA or acetate are the counter-ion, nitrogen is used because it is found in the peptide but not in TFA or acetate. Carbon or Nitrogen can be used used when chloride is the counter-ion for the same reason.
Net Peptide Weight calculations:
Net Peptide Quantity (total peptide) — method we use for net custom peptide orders.
Multiply the Net Peptide Content Percentage (decimal form) by the Peptide Gross Weight, to obtain the amount of Net Peptide Quantity (total peptide).
For example:
For 5 mg gross peptide/vial:
Peptide net content is 84%.
Exact amount of total peptide = 0.84*5 mg=4.2 mg total peptide
Net Peptide of Interest Quantity—most accurate — customer can use this method on their own to calculate peptide of interest.
Multiply Net Peptide Content Percentage by the Peptide Gross Weight, and the Peptide Purity (by HPLC), to calculate the exact amount of the peptide of interest you have. The net peptide percent (decimal form) is multiplied by peptide purity (decimal form).
For example:
For 5 mg gross peptide/vial with:
Peptide net content is 84%.
Exact amount of peptide of interest is = 0.84*0.96*5 mg=4.03 mg
MOL Changes utilizes solid-phase Fmoc chemistry rather than BOC chemistry. Fmoc chemistry allows for the removal of protecting groups using mild conditions during peptide elongation, while BOC chemistry requires the use of a strong acid to remove the protecting groups during peptide elongation. Additionally, Fmoc chemistry utilizes trifluoroacetic acid (TFA) to remove the peptide from the resin, while BOC chemistry utilizes hydrofluoric acid (HF). HF is colorless, highly corrosive, and a contact poison.
Therefore, MOL Changes employs Fmoc chemistry as it is more mild, flexible, and versatile compared with BOC chemistry.
Molarity refers to the molar concentration of a solution, which is the number of moles of solute dissolved in 1 liter of solution, expressed as mol/L, or M.
Molarity [M] = Mass / (Volume x Molar Mass); Mole = Concentration (g/L) x Volume (L)/MW (g/mol)
Example:
Given: 1mg of dry peptide powder with MW: 20KDa (Molar mass of peptide is 20 g/mol)
To determine molarity with known mass and known volume
For a 1ml solution of this peptide:
Molarity = Mass (0.001g) / (volume (0.001L) x Molar Mass (MW 20,000) = 50μM
To determine mass to achieve a certain molarity:
If for your assay, you need 0.01mM working peptide solution in 1ml of water, then calculate mass required as follows:
Mass = Molarity x Volume x Molar Mass
Mass = 0.01mM x (1/1000 L) x 20,000g/mol = 0.0002g
Hence, you will need 0.2mg/ml of peptide to have a working solution of 0.01mM.
We deliver as lyophilized or raw powder and peptide solution.
The solubility of a peptide in a given solvent is dependent on the amino acid sequence and often hard to predict. In the case of catalog peptides, the solubility information is listed on the QC datasheet. For custom peptides, please see the section entitled "How should I solubilize my peptide".
Peptide content is not an indication of the peptide purity as these are two separate measurements. Purity is determined by analytical HPLC and indicates the presence/absence of other peptidic contaminants (i.e. truncated peptides).
Net peptide content determines the actual amount of the peptide (peptide of interest as well as the peptide contaminants) present in the sample. The net peptide content is traditionally measured by Amino acid analysis (AAA; limited accuracy but requires a low amount of material) or elemental analysis (CHN; requires milligrams of peptide but is more accurate).
MOL Changes peptides are generally purified by reverse-phase preparative HPLC, using 2 buffers containing 0.1% trifluoroacetic acid (TFA). The first buffer (Buffer A) is composed of 0.1 % TFA in deionized water and the second buffer (Buffer B) is composed of 0.1 % TFA in acetonitrile (ACN).
Generally, crude peptides are first dissolved in a solution of either Buffer A, some amount of Buffer B, or an organic polar solvent such as DMSO. If an organic polar solvent or Buffer B was used, this clear solution will first be diluted with Buffer A. Next, the peptidic solution is filtered and loaded onto the preparative HPLC system. Utilizing an optimized method gradient, fractions are then collected based on the absorbance readings. Each fraction is then analyzed via an analytical HPLC to ensure that the purity of each fraction meets the required purity specification.
These “pure” fractions are then pooled together and lyophilized. The “pure” powder is then tested for both purity and identity to ensure that the correct peptide has been manufactured.
Finally, an independent QC group at AnaSpec again tests the peptide to ensure that all of the testing attributes meet the required specifications.
The international nomenclature used for the sequence termini is as follows:
- N-terminus: H means free amine (NH2-), Ac means acetyl [CH3C(O)-NH-], Pyr means pyroglutamic acid
- C-terminus: OH means free acid (-COOH), NH2 means amide [-CONH2]
- Modifications on the side chain of amino acids are depicted in the parenthesis after the corresponding amino acid. For example; phosphorylated serine = S(PO3H2) or epsilon-N-acetylated lysine = K(Ac)
在肽生产过程中,合成和纯化溶液中的离子会与肽带电的氨基酸侧链结合,形成盐。这些离子被称为肽反离子,有助于平衡肽的电荷。
三氟乙酸(TFA)常用于肽的合成和纯化。
它与碱性氨基酸(例如赖氨酸、精氨酸、组氨酸)和游离的N端胺形成三氟乙酸盐。因此,三氟乙酸盐往往是默认的反离子。
与肽结合的TFA分子的最小数量与给定肽序列中碱性残基的数量成正比。因此,肽中存在的碱性残基数量越多,冻干粉的净肽含量就越低。为了将样品中存在的TFA或水的含量考虑在内,我们建议对每个定制肽都进行肽含量测定。
水和三氟乙酸 (TFA) 是纯化过程中的副产物。然而,TFA 可能会对 体内和临床前研究造成毒性担忧。因此,MOL Changes 提供无毒的醋酸盐、氯化物或铵盐交换剂。
Peptides containing Cys, Met, or Trp are challenging to synthesize and obtain high-purity products. This is mainly due to the instability and susceptibility to oxidation of these functional groups. Special attention is required for the use and storage of these peptides to avoid repeated opening of the vials.
Did we miss your question? Drop us a line and we will
get back within 24 hours.

MOL Changes is a research and development organization specializing in the field of peptide science and is committed to providing multiple viable research avenues for the advancement of peptide science.
Get in Touch
- WhatsApp:+86 183 7515 8599
- Email:info@molchanges.com
- Mon-Fri 08:00 AM - 08:00 PM
- No. 58, Airport Avenue, Changsha
© 2023 MOL Changes Biotechnology Co., Ltd. All Rights Reserved.
- Home
- Terms & Conditions
- Privacy Policy
- Sitemaps