Peptide Modifications
Stapled Peptides
What is Stapled Peptides?
Studies have shown that peptides with α-helical structure and rich in positive charge can cross cell membranes. However, once separated from the parent body, the peptide cannot maintain its original secondary structure, and the conformational instability leads to the weakening of its binding to proteins, whereas ordinary linear peptides cannot cross the cell membrane and are easily hydrolysed. After continuous attempts, Verdine et al. developed a new structure of peptide, this peptide is called stapled peptide, it is an all-carbon scaffold with α-helical structure, all-carbon scaffold stabilises the α-helical structure, which strengthens the interaction between peptide molecules and proteins, and stapled peptide can cross the cell membrane and is not easy to be hydrolysed, which has a higher pharmacological activity compared with the previous small-molecule drugs and protein-based drugs. higher pharmacological activity than previous small molecule drugs and protein drugs.
Stapled Peptides science
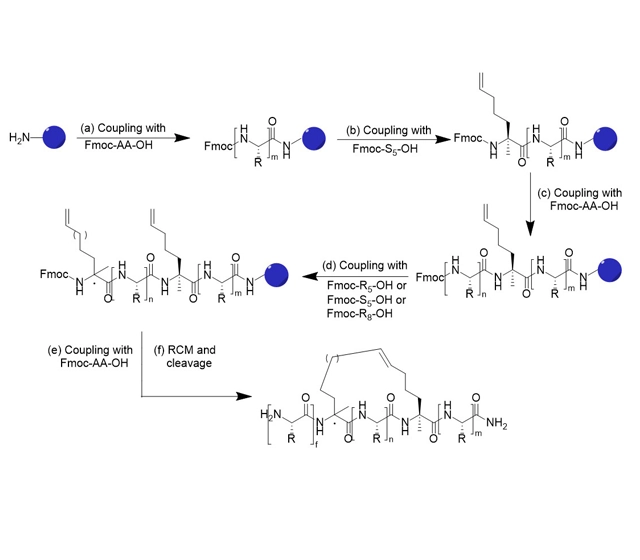
The difference between the synthesis of stapled peptide and ordinary peptide synthesis lies in the introduction of two non-natural amino acids containing α-methyl and α-alkenyl groups during the solid-phase synthesis of the peptide chain, and then the olefinic complex decomposition reaction occurs between the two non-natural amino acids to form an all-carbon scaffold with stable α-helical conformation, and then synthesize the stapled peptide.
Stapled Peptide Chemistry
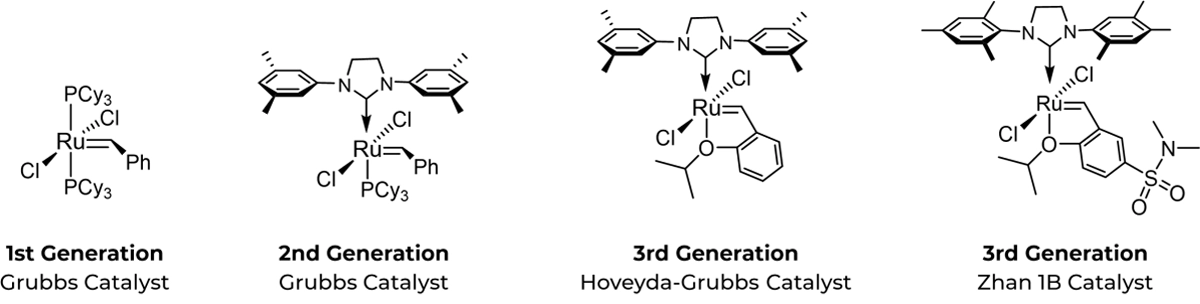
The first-generation Grubbs catalyst (left) with tricyclohexylphosphine (PCy3) ligands and apical positioned carbene carbon is a relatively stable ruthenium complex used for olefin metathesis in peptides. Subsequent investigations led to the design of a more thermally stable Grubbs second-generation catalyst (middle). A 3rd generation catalyst, also known as the Hoveyda–Grubbs catalyst (right), replaces the N-heterocyclic carbene ligand for a benzylidene ligands that have a chelating ortho-isopropoxy group attached to the benzene ring.
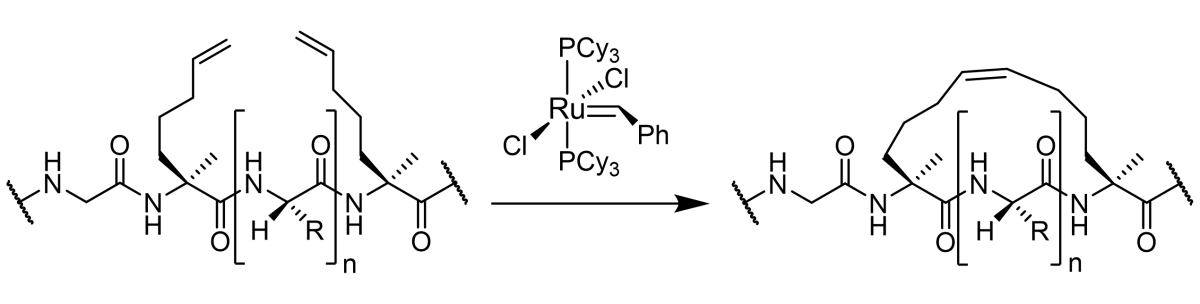
Single stapled peptide reaction
Single stapled peptide reaction. The incorporation of two alpha-4-n-pentenylalanine (S5) residues into a peptide strand enables ring-closure metathesis (i.e., Grubbs reaction) to create a single stapled peptide. When n = 3 (i.e. with 3 amino acids between the S5 residues) the staple type is known as an i, i + 4.
Single Staple Configurations
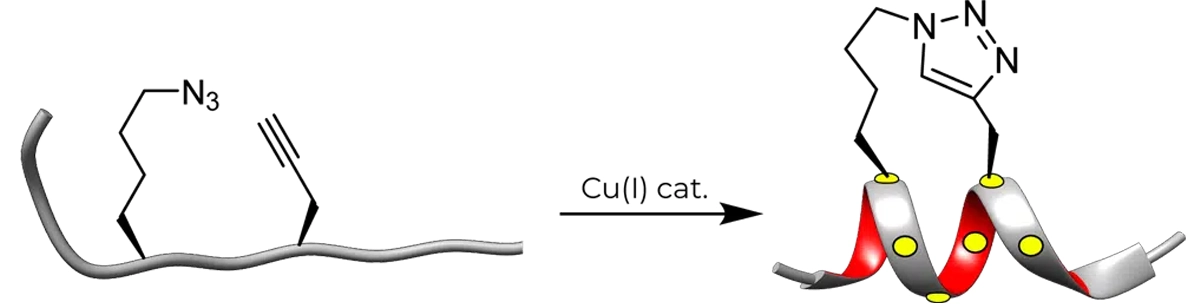
The high efficiency and mild conditions of “click” reaction (Copper-catalyzed Huisgen 1,3-dipolar cycloaddition reaction) combined with the ease of synthesis of the necessary unnatural amino acids, allows for facile synthesis of triazole-stapled peptides. For example, a combination of L- Nle (εN3) and D-Pra (D-propargylalanine) substituted at the i and i+4 positions can be used for the generation of single triazole-stapled peptides.
Featured Citations
Design-rules for stapled peptides with in vivo activity and their application to Mdm2/X antagonists.
Although stapled α-helical peptides can address challenging targets, their advancement is impeded by poor understandings for making them cell permeable while avoiding off-target toxicities. By synthesizing >350 molecules, we present workflows for identifying stapled peptides against Mdm2(X) with in vivo activity and no off-target effects. Key insights include a clear correlation between lipophilicity and permeability, removal of positive charge to avoid off-target toxicities, judicious anionic residue placement to enhance solubility/behavior, optimization of C-terminal length/helicity to enhance potency, and optimization of staple type/number to avoid polypharmacology. Workflow application gives peptides with >292x improved cell proliferation potencies and no off-target cell proliferation effects ( > 3800x on-target index). Application of these ‘design rules’ to a distinct Mdm2(X) peptide series improves ( > 150x) cellular potencies and removes off-target toxicities. The outlined workflow should facilitate therapeutic impacts, especially for those targets such as Mdm2(X) that have hydrophobic interfaces and are targetable with a helical motif.
Related Peptide Modification Services
peptide glycosylation
peptide glycosylation
Peptide glycosylation is a covalent modification that can potentially improve the physicochemical properties of peptides
Read Morepeptide Phosphorylation
peptide Phosphorylation
Phosphoralation may occur on Serine (S, Ser), Threonine (T, Thr) and Tyrosine (Y, Tyr) side chains by phosphoester bond formation
Read MoreStapled Peptides
Stapled Peptides
Introduction of two unnatural amino acids containing α-methyl, α-alkenyl groups during solid-phase synthesis of peptide chains.
Read MoreCyclic Peptide
Cyclic Peptide
Peptide cyclisation enhances the conformational stability of peptides (relative to their linear analogues) and is a common strategy in peptide development.
Read Morepeptide modification
Extensive experience in peptide modification, providing multiple viable avenues for peptide research.